24 Cardiac Transplantation and Mechanical Circulatory Support Devices
Donor Procedure
After all the organs are placed, procurement surgeons arrive at the donor hospital, and a coordinated procedure allows simultaneous procurement of all usable organs, often including the heart, lungs, liver, kidneys, and pancreas and occasionally including the small intestine. The heart explant procedure depends on whether the heart alone will be used or whether the lungs will also be used separately or as a combined heart-lung transplant. After initial dissection of the aorta and superior and inferior venae cavae, placement of a cardioplegia cannula in the ascending aorta, and completion of the other teams’ initial dissections, the donor is systemically heparinized. The superior vena cava is tied off, the left atrial (LA) appendage is amputated, and the inferior vena cava is partially transected to decompress the heart and prevent ventricular distention. The aorta is then cross-clamped, and cardioplegia is infused while the heart is lavaged with ice-cold saline (Fig. 24-1).
Recipient Procedure
Biatrial Technique
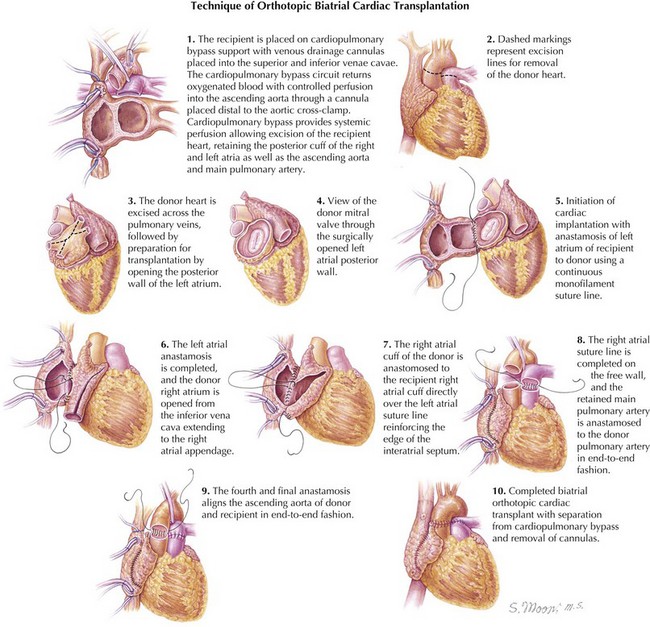
The operation is performed through a standard median sternotomy using cardiopulmonary bypass with aortic and bicaval cannulation. The initial dissection and cannulation are performed while the heart is being transported to the recipient hospital. When the new heart arrives, cardiopulmonary bypass is instituted at moderate systemic hypothermia (~32°C), and caval tapes are secured around the caval cannulas. The aorta is cross-clamped and then divided just above the level of the aortic valve. The pulmonary trunk is divided above its respective valve, and the atria are divided at the midatrial level, with removal of the atrial appendages and preservation of the posterior atrial cuffs containing the pulmonary veins on the left and the cavae on the right. The donor heart is prepared by freeing the pulmonary artery from the aorta and the roof of the left atrium. The pulmonary venous orifices are interconnected to create a cuff for the LA anastomosis. Excess LA tissue can be removed to create a better size match for this anastomosis. The oval fossa of the donor heart is examined for a patent foramen ovale. If identified, it is closed. The LA anastomosis is then fashioned with a suture in a continuous running fashion. The suture line is begun at the base of the donor LA appendage, just above the recipient left superior pulmonary vein (see Fig. 24-1).
Bicaval Technique
The operation is fundamentally the same as the biatrial technique. The differences in cardiectomy include developing the groove between the right and left atria to allow their separation. During excision of the heart, the superior vena cava is divided just above the level of the right atrium, and the inferior vena cava is divided just below the coronary sinus. After the aorta and pulmonary artery have been divided, a LA cuff is then created starting at the dome of the left atrium, carrying the incision inferiorly above the orifices of the right and left pulmonary veins (Fig. 24-2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree