CARDIC SURGERY IN THE NEONATE
Introduction
Cardiac surgery in the neonate has become an important and accepted treatment for many forms of structural heart disease. Although a detailed discussion of cardiac surgery is beyond the scope of this chapter, an overview is helpful for all practitioners who care for neonates, both to help participate in their care and to aid in counseling families. This chapter will be subdivided into 2 parts: general principles and a discussion of specific procedures. The procedures will itself be subdivided into palliative procedures and definitive repairs.
General Principles
A few general principles and definitions are helpful for all practitioners. The overview topics deal with techniques, timing, and other relevant definitions.
Techniques
Incisions Most cardiac surgical procedures are performed through a median sternotomy.1,2 A median sternotomy can be performed in a patient of virtually any age. It offers a wide exposure to cardiac structures so that there is flexibility to change the surgical plan intraoperatively. It is the ideal approach if the patient unexpectedly requires cardiopulmonary bypass (CPB). Even though the surgical scar is cosmetically prominent, there are no nerves or muscles divided, so it is an excellent functional incision. There are a few procedures that are performed through different incisions. Procedures involving the proximal descending aorta, such as ligation of patent ductus arteriosus (PDA) or repair of aortic coarctation, are performed through a left thoracotomy.1,2 A limitation of the left thoracotomy is that CPB cannot be performed through this approach. A right thoracotomy is the classical approach for a Blalock–Taussig (BT) shunt; BT shunts can also be performed via a median sternotomy.
CPB, aortic cross-clamping, and circulatory arrest CPB is mechanical support of the heart and/or lung function during a cardiac surgical procedure. For many procedures, CPB is necessary as the heart cannot be repaired while simultaneously maintaining perfusion and/or oxygenation to the body. CPB allows the heart to be decompressed while ensuring systemic perfusion during manipulation of the heart. CPB, however, comes with a price. The entire blood volume is diverted through a series of plastic tubes as well as an oxygenator. This process initiates a tremendous inflammatory response.3–6 For this reason, many of the palliative procedures are performed in lieu of corrective procedures in order to avoid CPB. In its simplest form, CPB consists of 2 cannulae: a venous cannula (often in the right atrium) and an arterial cannula (usually in the ascending aorta). Blood is siphoned from the venous circulation through the venous cannula into the CPB circuit. The blood is pumped through the oxygenator and then back into the arterial cannula, where it perfuses the body. If intracardiac surgery is required, venous cannulae can be placed directly in the inferior and superior vena cavae, such that a bloodless intracardiac field is obtained. If the heart needs to be made motionless, the middle of the ascending aorta is clamped. This isolates the coronary arteries from the arterial inflow. A small infusion catheter is inserted into the ascending aorta proximal to the aortic cross-clamp. A cold, hyperkalemic solution is infused into the proximal ascending aorta and into the coronary arteries.7,8 The myocardium is perfused with this cardioplegia solution, which renders the heart motionless by putting it into a diastolic arrest. Typical CPB times are 60 to 180 minutes, while cross-clamp times range from 30 to 120 minutes. These times can vary beyond these limits depending on the complexity of the procedure or the occurrence of intraoperative complications. Circulatory arrest is a technique that allows all perfusion to cease for key portions of a cardiac procedure. With circulatory arrest, the body is cooled to a point where tissues may survive for periods of time (up to 1 hour) without any perfusion. Typical core temperatures are reduced to 18 to 20°C. Circulatory arrest is indicated in 2 circumstances. One indication is that the arteries transporting the blood to portions of the body must be opened or occluded to perform the repair. The most typical circumstance is an aortic arch repair. If the inflow from the CPB machine cannot be rerouted around the repair site, then the surgeon may elect to cease all perfusion until that artery can be repaired. Circulation is then resumed. The other indication is if venous return cannot be properly controlled. An example of this may be removal of the atrial septum. Bicaval cannulation (described above) may be employed to allow the surgeon to open the heart. If the patient is not suitable for bicaval cannulation, the surgeon may elect to cease the circulation during the removal of the septum.
Timing of surgery Most neonatal cardiac surgical procedures are performed between 4 and 14 days of age.9 The majority of congenital defects requiring neonatal surgery are dependent on ductal patency, which is maintained by prostaglandin E1 (PGE1) infusion.10,11 As patients can be stabilized with PGE1, surgery for these lesions is not emergent and need not be performed in the first several days of life. This has advantages beyond scheduling concerns. The newborn infant undergoes a significant stress response at the time of delivery. Waiting several days allows the stress response to subside. Pulmonary vascular resistance drops over the first few days of life, improving outcomes from cardiac surgery.12,13 Cardiac surgical procedures typically done in the 4 to 14 day window include BT shunts, Norwood procedures, interrupted arch repairs, critical coarctation repairs, and arterial switch procedures.9,14–17 Some procedures are not ductal dependent and can be performed within or beyond the 14 day window, such as repair of truncus arteriosus (TA). Pulmonary artery (PA) banding, performed for patients with excessive pulmonary blood flow (PBF), is generally done from 2 to 6 weeks of age when pulmonary vascular resistance has had a chance to decrease more than what occurs in the first 14 days of life. Even in the current era, there are procedures with varying practice patterns for timing. Unobstructed total anomalous pulmonary venous return (TAPVR) is performed within the first 2 weeks of life in some institutions. In other institutions, these patients are discharged and performed semielectively.
The procedure that constitutes the classic pediatric cardiac surgical emergency is obstructed TAPVR.18,19 In other words, the anomalous pulmonary venous return is obstructed along the path between the lungs and the eventual return to the right atrium. The pulmonary venous pressure is high, resulting in pulmonary edema. There is no medical (noninvasive) palliation for obstructed pulmonary venous return and therefore, severely obstructed TAPVR must be repaired emergently.
Very occasionally, there are miscellaneous situations that may require emergency surgery, whether it be actual cardiac surgery or support with extracorporeal membrane oxygenation (ECMO). An example may be a patient with pulmonary atresia who has a restrictive (or atretic) PDA. A patient with this morphology would have no source of PBF and may require an emergency BT shunt. However, these situations are rare and cardiac surgery performed in the first 72 hours of life is uncommon. Comorbidities such as intracranial hemorrhage, necrotizing enterocolitis, or extreme prematurity may delay cardiac procedures beyond the typical 4 to 14 day window, and such decisions must be individualized.
Specific Lesions and Procedures
Certain specific cardiac procedures may be performed for different cardiac diagnoses. For example, a BT shunt may be performed for single-ventricle lesions (eg, double-inlet left ventricle [DILV]) or biventricular lesions (eg, tetralogy of Fallot [TOF] with severe pulmonary stenosis [PS]). Other repairs are performed only for specific lesions. “Repair of TA” is considered a specific procedure, but is clearly performed only for patients with TA. It is more advantageous to discuss palliative procedures as organized by procedure. Corrective procedures are better organized by diagnosis.
Palliative versus Corrective
The definition of palliative versus corrective (also known as “complete repair”) deserves clarification. A corrective procedure is one that restores normal biventricular physiology. In other words, blood from the vena cavae returns to the right heart, is pumped to the lungs, returns to the left heart and is pumped to the body, and then returns to the vena cavae. The cycle then repeats. Common examples of complete repairs include repair of TOF and repair of TA. “Corrective repairs” (or “complete repairs”) may still leave significant residual abnormalities including valve insufficiency, diastolic dysfunction, or the presence of conduits that do not grow. The term “corrective” (or complete repair) is somewhat misleading in these situations. Corrective procedures are often far from restoring a normal heart, but are aimed at optimizing cardiac physiology within the anatomic constraints of each congenital defect. Palliative procedures, on the other hand, do not restore the above-mentioned normal physiology. Palliative procedures may increase PBF, decrease PBF, relieve systemic outflow obstruction, etc., but they do not achieve normal biventricular physiology. Single-ventricle procedures are, by definition, palliative since biventricular physiology cannot be achieved. Palliative procedures can also be applied to diagnoses, which eventually get corrective procedures; for example, BT shunts can be used to palliate TOF in children who eventually get a complete repair.20
Palliative Procedures for Single-Ventricle Physiology
Palliative procedures are often performed for patients with single-ventricle physiology (SVP). A thorough discussion of the surgical management strategies for single-ventricle lesions is discussed in Chapter 18. In brief, children with SVP require unobstructed blood flow to the body and modest restriction of blood flow to the lungs. This is derived from the following facts:
- The blood flow from the single ventricle takes a “fork in the road” where some of the blood goes to the body, and some goes to the lungs.
- Pulmonary vascular resistance is less than systemic vascular resistance.
- The desired quantity of blood flow to the body and to the lungs should be roughly equal.21,22
Given this situation, children with SVP require unobstructed blood flow to the body and a modest obstruction of blood flow to the lungs. Unobstructed blood flow to the lungs will result in pulmonary over-circulation given the low pulmonary vascular resistance. Severe obstruction to the lungs will result in cyanosis from lack of PBF. Any obstruction to systemic blood flow (SBF) adds an unnecessary afterload on the heart and likely limits necessary systemic circulation. Thus, children with SVP are managed with the following procedures:
| SBF | PBF | Procedure | Typical example |
| Unobstructed | Unobstructed | PA band | DILV with unobstructed great vessels |
| Unobstructed | Modest obstruction | No procedure needed | Tricuspid atresia with modest pulmonary outflow obstruction |
| Unobstructed | Severe obstruction | BT shunt | Pulmonary atresia |
| Obstructed | Unobstructed | Norwood | Hypoplastic left heart syndrome (HLHS) |
Specific Palliative Procedures
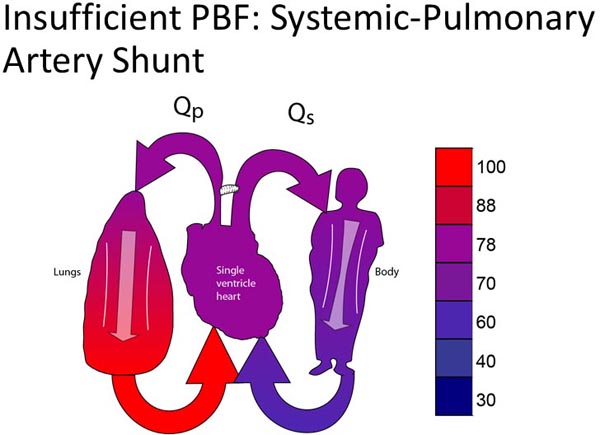
Systemic pulmonary artery (SPA) shunt An SPA shunt is a connection between the aorta (or branch thereof) and the PA.20,23 The most common form of an SPA shunt is a modified BT shunt, which is a polytetrafluoroethylene (PTFE) vascular graft connecting the innominate and right pulmonary arteries (RPAs).23 The classic BT shunt is performed by dividing the subclavian artery and directly connecting it to the RPA, but is rarely performed in the current era.20 A central SPA shunt is performed by connecting a PTFE graft from the ascending aorta to the PA. In general, SPA shunts can be performed without the use of CPB. The avoidance of CPB in the neonatal period is felt to be one of the potential advantages of palliation over complete repair. Some babies do not tolerate the surgical manipulation required for an SPA shunt and will require the support of CPB. The mortality of SPA shunts is higher than what might be expected from a procedure that is relatively short and limited in magnitude. SPA shunts are very technically demanding, and leave little margin of error in terms of creating a conduit that provides enough PBF without exposing the patient to pulmonary overcirculation. Appropriate shunt flow depends not only on the technique of the shunt placement but also on the relative balance of the pulmonary and systemic vascular resistance. The real possibility of inappropriate PBF contributes to the mortality of the procedure. In addition, there is the inherent risk of shunt thrombosis. The exact risk of shunt thrombosis is unknown, but is roughly 2% to 4%. Patients with an SPA shunt also have runoff from the systemic circulation, through the shunt, and into the pulmonary circulation in diastole. Thus, patients with SPA shunts have low diastolic pressures, which may compromise coronary perfusion. A transient decrease in cardiac output from hypovolemia or decreased function may cause an ischemic catastrophe from decreased coronary perfusion and rapidly results in a cardiac arrest.
Figure 25.1. Schematic diagram showing SPA shunt for patient with insufficient PBF with single ventricle.
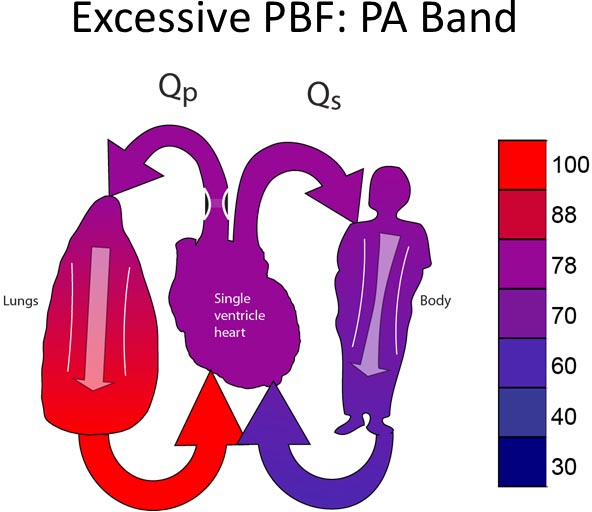
PA banding PA banding (Figure 25.2) is performed for patients with excessive PBF. Typically, PA banding is done in one of the three clinical scenarios:
- PA banding is performed on patients with SVP and unobstructed SBF and unobstructed PBF.
- PA banding may be performed to palliate patients who require complicated biventricular repairs, but are suffering from heart failure from excessive PBF. Because of the complexity of the definitive repair, PA banding can be done to relieve the heart failure and defer the procedure until the patient is bigger/older. Typical examples may be patients with double outlet right ventricle or multiple ventricular septal defects (VSDs).
- PA banding may also be done to reduce heart failure in a child, who requires a more straightforward biventricular repair (eg, VSD closure, atrioventricular canal repair), but suffers from comorbidities that preclude definitive repair with CPB (eg, refractory respiratory viral illnesses, intracranial hemorrhage). Placement of a PA band allows for immediate treatment of the heart failure with definitive repair deferred until the comorbidities have resolved.
PA banding is uncommonly performed in the neonatal period. PA banding is more often performed when patients’ pulmonary vascular resistance falls to the point where the patient has symptoms of heart failure or their pulmonary vasculature requires protection from high PA pressures.24–26 Although this may be occasionally done as a neonate, it is more typically performed in the second month of life. PA banding virtually never requires the use of CPB. Unlike SPA shunts, there is no diastolic run-off. In this sense, the physiology of a patient after a PA band is more favorable than after an SPA shunt.
Figure 25.2. PA band for patient with excessive PBF.
Damus-Kaye-Stansel (DKS) and Norwood procedure (Figure 25.3) A DKS procedure is done for neonates with obstructed SBF and unobstructed PBF.27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree