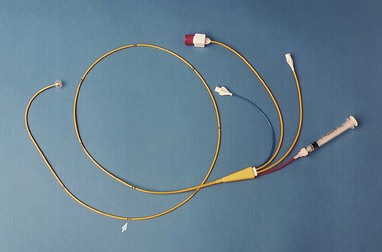
Charles J. Davidson, Robert O. Bonow The decision to recommend cardiac catheterization is based on an appropriate risk-benefit ratio. In general, diagnostic cardiac catheterization is recommended whenever it is clinically important to define the presence or severity of a suspected cardiac lesion that cannot be evaluated adequately by noninvasive techniques. Because the risk for a major complication from cardiac catheterization is less than 0.5% and mortality is less than 0.08%, there are few patients who cannot undergo the procedure safely in an active laboratory. Intracardiac pressure measurements and coronary arteriography are procedures that can be performed best with reproducible accuracy by invasive catheterization. Alternatively, intracardiac pressures can be estimated noninvasively with echocardiography (see Chapter 14). Coronary computed tomography (CT) angiography can also be used for assessment of coronary anatomy (see Chapter 18) and provides adjunctive information on plaque distribution and composition. However, limitations of spatial resolution, heart rate variability, patient cooperation, and radiation dosing limit the ability of CT to replace cardiac catheterization for definition of coronary artery stenosis. To understand the various indications for diagnostic cardiac catheterization, integration of knowledge from multiple American College of Cardiology/American Heart Association (ACC/AHA) guidelines is necessary.1–9 These guidelines address specific indications for cardiac catheterization related to disease states, including guidelines for the management of patients with valvular heart disease,1 chronic heart failure,2 ST-elevation myocardial infarction (STEMI),3 percutaneous coronary intervention (PCI)4 and coronary artery bypass grafting (CABG),5 unstable angina or non-STEMI,6 and congenital heart disease.7 Cardiac catheterization is indicated in diverse populations. At one extreme, many critically ill and hemodynamically unstable patients are evaluated during acute coronary syndromes, severe heart failure, or cardiogenic shock. At the other end of the spectrum, many procedures are performed in an outpatient setting. Such settings include hospitals with or without cardiac surgical capability and freestanding or mobile laboratories.9 Cardiac catheterization should be considered a diagnostic study used in combination with complementary noninvasive tests. For example, cardiac catheterization in patients with valvular or congenital heart disease is best performed with full prior knowledge of any noninvasive imaging and functional information. This allows catheterization to be directed and simplified without obtaining redundant anatomic information that is reliably available with echocardiography, cardiac magnetic resonance (CMR) (see Chapter 17), or CT. Identification of coronary artery disease and assessment of its extent and severity are the most common indications for cardiac catheterization in adults. The information obtained is crucial to optimize selection of mechanical or medical therapy. In addition, dynamic coronary vascular lesions, such as spasm, myocardial bridging, and plaque rupture with thrombosis, can be identified. The consequences of coronary heart disease, such as ischemic mitral regurgitation and left ventricular (LV) dysfunction, can also be defined. During PCI for acute coronary syndromes, patients are studied during evolving acute myocardial infarction, with unstable angina, or in the early period after acute myocardial injury. The optimal timing for catheterization and revascularization has been described in various guidelines3,4,6 (see Chapters 52 and 53). In patients with myocardial disease and LV dysfunction, cardiac catheterization provides important hemodynamic and coronary artery information. It can be used to evaluate the severity of coronary artery disease and quantify LV and right ventricular (RV) hemodynamics and function. In patients with angina and impaired LV function, noninvasive testing has limitations and coronary angiography is often indicated to differentiate ischemic from nonischemic cardiomyopathy.2 Cardiac catheterization also permits quantification of the severity of both diastolic and systolic dysfunction and differentiation of myocardial restriction from pericardial constriction. In patients with valvular heart disease, cardiac catheterization is both confirmatory of and complementary to findings on echocardiography and CMR (see Chapter 63). Cardiac catheterization can define the severity of valvular stenosis or regurgitation, particularly when noninvasive studies are inconclusive or the results are disparate from the clinical findings. Knowledge of coronary artery anatomy is necessary in most adults older than 35 years when valve surgery is planned.1 However, catheterization may be unnecessary in some preoperative situations, such as younger patients (<55 years) with atrial myxoma, endocarditis, or acute valvular regurgitation. Identification of congenital anomalies, quantification of the hemodynamic consequences of valvular lesions (such as pulmonary hypertension), and the acute hemodynamic response to pharmacologic therapy can provide useful preoperative information that helps define the risk and response to surgery and permits a more directed surgical approach.1 The current role of cardiac catheterization in certain congenital disease states has been addressed in guidelines for adults with congenital heart disease7 (see Chapter 62). Echocardiography with Doppler and CMR often provide adequate information. Because gross cardiac anatomy can generally be well defined by these methods, catheterization is required only if certain hemodynamic information (e.g., quantification of shunt severity, pulmonary vascular resistance [PVR], and reversibility of pulmonary arterial hypertension with a vasodilator) is needed for confirmation in determining the indications for surgical procedures or if percutaneous interventions are being considered. There is no true absolute contraindication to cardiac catheterization other than refusal by a competent patient. The procedure can be performed successfully with relatively low risk even in the most critically ill patients. Relative contraindications to cardiac catheterization are summarized in Table 19-1. TABLE 19-1 Relative Contraindications to Diagnostic Cardiac Catheterization Before arrival in the catheterization laboratory, the cardiologist responsible for the procedure should explain the procedure fully, including the risks and benefits, and answer questions from the patient and family. Precatheterization evaluation includes a patient history, physical examination, and ECG. Routine laboratory studies include a complete blood count with platelets, serum electrolyte determinations with creatinine and estimated glomerular filtration rate (eGFR), prothrombin time with international normalized ratio (INR) (in patients receiving warfarin or with hepatic disease), and the partial thromboplastin time (in patients receiving heparin). Important components of the history that need to be addressed include diabetes mellitus (insulin or non–insulin requiring), kidney disease, anticoagulation status, peripheral arterial disease, and previous allergy to contrast media or latex. Full knowledge of any previous procedures, including cardiac catheterizations, PCI, peripheral arterial interventions or surgery, and cardiac surgery, is necessary. Patients should be fasting for at least 6 hours, and an intravenous line should be established. Oral or intravenous sedation is often administered (e.g., benzodiazepine). Pulse oximetry should be used to monitor respiratory status. Warfarin should be discontinued approximately 3 days before and the INR should be less than 1.8 to minimize risk for bleeding. An INR lower than 2.2 is acceptable for radial artery access.9 In patients receiving dabigatran, use of the medication should be discontinued 24 hours before catheterization in patients with normal renal function and 48 hours before in those with an eGFR higher than 30 and lower than 50 mL/min. A lower eGFR will require several days of cessation. Aspirin and/or other oral antiplatelet agents are continued before the procedure. Patients with diabetes receiving metformin should have use of the medication discontinued the morning of the procedure and not be restarted until renal function is stable for at least 48 hours after the procedure.11 To minimize the risk for contrast-induced nephropathy, all patients should receive hydration before and after the procedure. The amount of hydration is dependent on LV function and baseline fluid status. However, if tolerated, a total of 1 liter of normal saline administered between initiation and completion of the procedure is recommended. Another hydration regimen that has been studied to prevent contrast-induced nephropathy in patients with chronic kidney disease is the use of sodium bicarbonate at 3 mL/kg for 1 hour before the procedure and 1 mL/kg for 6 hours after.12 This regimen was initially reported to be superior to normal saline, but recent data have shown equivalence. Despite this lack of superiority, it is a simple and rapid regimen for prevention of contrast-induced nephropathy. Those with a previous history of allergy to contrast media need prophylaxis before the procedure.13 A recommended regimen is the administration of either prednisone (50 mg by mouth) or hydrocortisone (100 mg by intravenous push) 12 hours and immediately before the procedure. Cimetidine (300 mg by intravenous push or by mouth), a nonselective histamine antagonist, and diphenhydramine (25 to 50 mg by intravenous push) may also be given. A common misconception is that a history of shellfish allergy predisposes patients to contrast media reactions. Tropomyosin, not the iodine in shellfish, appears to be the allergen. A general routine for performing diagnostic catheterization will ensure efficient acquisition of all pertinent data. In general, hemodynamic measurements and determination of cardiac output should be done before angiography to reflect the basal conditions most accurately. However, in a high-risk case, the approach is to acquire the most important information first because of the possibility of patient instability. Right-heart catheterization should not be performed in all patients undergoing routine coronary angiography because of the low yield in those with suspected coronary artery disease without other known cardiac disease. Right-heart catheterization should include screening oximetric analysis, measurement of intracardiac pressures, and determination of cardiac output. Right-heart catheterization is indicated when a patient has LV dysfunction, heart failure, complicated acute myocardial infarction, valvular heart disease, suspected pulmonary hypertension, congenital heart disease, intracardiac shunts, or pericardial disease. Although use of a temporary pacemaker is not indicated for routine cardiac catheterization, operators should understand the techniques for proper insertion. Even in patients with an isolated left bundle branch block, right-heart catheterization can generally be performed safely with balloon flotation catheters without causing any additional conduction disturbance. An example of a balloon flotation catheter (Swan-Ganz) is shown in Figure 19-1. Catheters used for cardiac catheterization are available in various lengths, sizes, and configurations. Typical catheter lengths vary between 50 and 125 cm, with 100 cm being used most commonly for adult left-heart catheterization via the femoral approach. In patients with a dilated ascending aorta or tortuous ascending or descending aorta, a longer 125-cm catheter is often used. The outer diameter of the catheter is specified in French units, with 1F equaling 0.33 mm. The inner luminal diameter of the catheter is smaller than the outside diameter because of the thickness of the catheter material. Guidewires used during the procedure must be the proper caliber to pass through the inner diameters of both the introducer needle and the catheter. Guidewires are described by their length in centimeters, diameter in inches, and tip conformation. A commonly used wire is a 150-cm, 0.035-inch J-tip wire. Introducer sheaths are specified by the French number of the largest catheter that can pass freely through the inner diameter of the sheath rather than the outer diameter. Therefore a 7F introducer sheath accepts a 7F catheter (7F = 2.31 mm) but has an outer diameter greater than 7F. Selection of the size of the catheters to be used is determined by balancing the need to opacify the coronary arteries and cardiac chambers adequately and to permit sufficient manipulation of the catheter while limiting vascular complications and allowing earlier ambulation. The most commonly used catheters are 4F to 6F, which permit early ambulation after femoral artery access and generally provide adequate visualization. Smaller catheters require greater technical skill for manipulation and have lower flow rates. Thus their use in patients with tortuous anatomy, large body habitus, or high coronary flow states (e.g., aortic regurgitation) can be challenging. The relationship between sheath size and vascular complications is not clear within the range used for routine diagnostic catheterization. Rather, the arterial puncture technique, anticoagulation status, including the use of thienopyridines and glycoprotein IIb/IIIa receptor inhibitors, and the presence of coagulopathies are more important factors related to vascular complications.14
Cardiac Catheterization
Indications for Diagnostic Cardiac Catheterization

Technical Aspects of Cardiac Catheterization
Catheterization Laboratory Protocol
Preparation of the Patient for Cardiac Catheterization
Catheterization Protocol
Catheters and Associated Equipment
Right-Heart Catheterization
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cardiac Catheterization
19