, Ik-Kyung Jang2 and Ik-Kyung Jang3
(1)
Harvard Medical School Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School, Boston, USA
(3)
Cardiology Laboratory for Integrative Physiology and Imaging: CLIPI, Interventional Cardiology, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
Cardiac catheterization is currently the gold standard diagnostic procedure for assessing cardiac function and coronary anatomy. It is also a platform for the treatment of coronary artery disease peripheral vascular disease and, increasingly, structural heart disease.
Abbreviations
ACS
Acute coronary syndrome
AI
Aortic insufficiency
AP
Anterior posterior
AS
Aortic stenosis
AV
Aortic valve
AVA
Aortic valve area
BSA
Body surface area
CABG
Coronary artery bypass graft
CAD
Coronary artery disease
CIN
Contrast induced nephropathy
CO
Cardiac output
CVA
Cerebrovascular accident
CVP
Central venous pressure
DFP
Diastolic flow period
DM
Diabetes mellitus
FFR
Fractional flow reserve
HF
Heart failure
HOCM
Hypetrophic obstructive cardiomyopathy
HR
Heart rate
IVC
Inferior vena cava
IVUS
Intravascular ultrasound
LA
Left atrium
LAD
Left anterior descending artery
LAP
Left atrial pressure
LAO
Left anterior oblique
LCx
Left circumflex artery
LIMA
Left internal mammary artery
LM
Left main
LVEDP
Left ventricular end diastolic pressure
MAP
Mean arterial pressure
MR
Mitral regurgitation
MV
Mitral valve
MVA
Mitral valve area
MVO2
Mixed venous oxygen saturation
NSTEMI
Non-STE-elevation myocardial infarction
OCT
Optical coherence tomography
PA
Pulmonary artery
PAO2
Pulmonary artery oxygen saturation
PAWP
Pulmonary artery wedge pressure
PBF
Pulmonary blood flow
PCI
Percutaneous coronary intervention
PDA
Posterior descending artery
PHT
Pulmonary hypertension
PVO2
Pulmonary venous oxygen saturation
PVR
Pulmonary vascular resistance
RA
Right atrium
RAO
Right anterior oblique
RCA
Right coronary artery
RV
Right ventricle
RVEDP
Right ventricular end diastolic pressure
SBP
Systemic blood flow
SCD
Sudden cardiac death
SEP
Systolic ejection period
STEMI
ST-elevation myocardial infarction
SVC
Superior vena cava
SVG
Saphenous vein graft
VO2
Oxygen consumption
Introduction
Cardiac catheterization is currently the gold standard diagnostic procedure for assessing cardiac function and coronary anatomy. It is also a platform for the treatment of coronary artery disease, peripheral vascular disease and, increasingly, structural heart disease.
Cardiac Catheterization
1.3 million percutaneous coronary interventions (PCI) and 1.1 million diagnostic catheterizations performed in annually in U.S.
Contraindications (all relative): active gastrointestinal bleeding, coagulopathy (INR >1.8), acute renal failure, acute stroke, untreated infection, anemia, anaphylactoid contrast allergy, intracranial hemorrhage.
Radiation Safety [1]
Dose dependent effects of radiation: skin injury, cataracts, hair loss
Typical dose 3–5 mSv. Goal dose is as low as reasonably achieved
Stochastic effect: cancer, genetic defects.
Operator exposure is predominantly from scatter off of patient.
Energy of radiation decreases with square root of distance.
Arterial Access
Femoral artery most common access site in U.S. 3 % performed from radial artery but increasing rapidly [2].
Advantage of radial access: ↓70 % in major bleeding versus femoral; ↑ambulation time and ↑patient comfort. Possible ↓ in mortality with ST-elevation myocardial infarction (STEMI) [3].
Limitations of radial access: technically challenging, ↑procedure time (↑radiation and contrast). Two percent procedure failure rate.
Brachial access: similar complication rate as femoral access. Rarely used.
Left Heart Catheterization
Use of anticoagulation indicated for prolonged cases (e.g. previous coronary artery bypass graft (CABG) and when crossing stenotic aortic valve (AV)).
(A)
Coronary angiography (see below):
(B)
Left Ventriculography (Figs. 9-1–9-4):
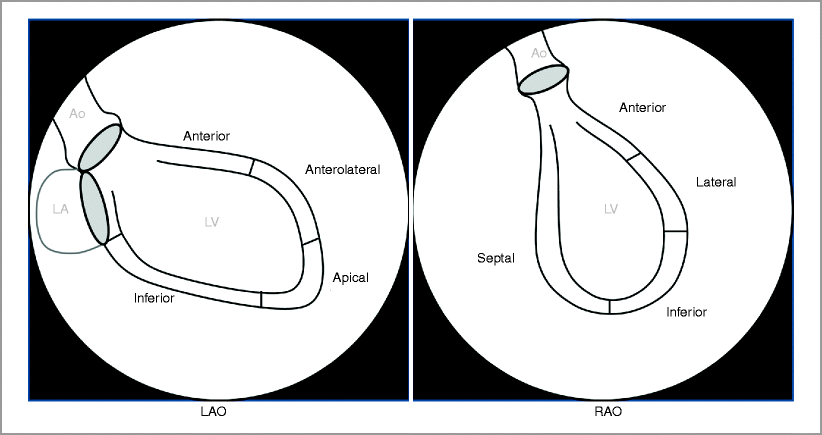

Figure 9-1
Left ventriculogram. Ao aorta, LA left atrium, LV left ventricle
Left ventriculography by power injection of 30–40 ml of contrast at 10–15 mL/s:
Right anterior oblique (RAO) shows anterior, anterolateral, apical and inferior/posterior walls
Left anterior oblique (LAO) shows anterior, lateral, inferior and septal walls
Left ventriculogram contraindicated if left ventricular end diastolic pressure (LVEDP) >25 mmHg or in presence of critical left main coronary artery stenosis (LMCA) stenosis.
Findings
Wall motion assessment
Ejection fraction
Presence of ventricular septal defect
Presence of aneurysm
True left ventricular (LV) aneurysms have broad neck and are associated with akinetic wall. Pseudoaneurysms have narrow necks.
Assessment of valvular regurgitation
Sellar’s criteria: (1) partial filling of proximal chamber, (2) complete filling of proximal chamber but less dense than distal chamber, (3) equal opacification of proximal chamber in 4–5 beats, (4) equal opacification of proximal chamber in ≤3 beats.
(C)
Pressure assessment in left heart catheterization
AV gradient assessed by simultaneous aortic and LV pressures (e.g. double lumen pigtail catheter). Indicated when non-invasive results inconclusive or discordant with clinical assessment (Class I recommendation). Not indicated when non-invasive results are conclusive (Class III)
Mitral valve (MV) gradient assessed by simultaneous LV and pulmonary capillary wedge pressure (PCWP) or left atrial (LA) pressure
(D)
Transseptal Catheterization:
Indications: assessment of native or prosthetic mitral valve stenosis (PCWP overestimates transmitral gradient), mitral valve commissurotomy, pulmonary vein isolation (electrophysiology), percutaneous mitral valve valvuloplasty, assessment of LVEDP in presence of mechanical AV, hypertrophic obstructive cardiomyopathy (Fig. 9-2).
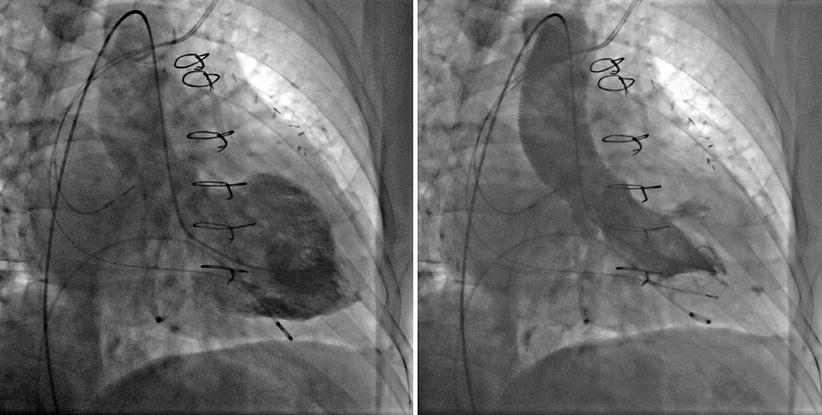
Figure 9-2
Apical hypertrophic cardiomyopathy in RAO view
Complications: puncture of atrial free wall, aortic root, coronary sinus, or pulmonary artery (PA).
(E)
Complications of Left Heart Catheterization [4]:
Most commonly access site related (<1 % of diagnostic procedures versus 5–20 % of PCIs).
Risk factors for access site complications: age, female sex, smaller body surface area, emergent procedures, multi-vessel coronary artery disease (CAD), anti-platelet therapy, coagulopathy, renal dysfunction, liver dysfunction, pre-existing peripheral arterial disease and use of larger sheaths [5].
Vascular complications: hematomas, retroperitoneal bleeding, pseudoaneurysm, atriovenous fistula, infection.
Retroperitoneal bleed: hypotension or back pain post procedure
CT scan is recommended diagnostic modality
Pseudoaneurysm
Treated with manual compression (small) or ultrasound guided thrombin injection (large)
Cerebrovascular Accident (0.07 %): MRI shows cerebral embolic events in 22 % of patients after crossing severe aortic valve → majority asymptomatic [6].
Other: contrast induced nephropathy (CIN), myocardial infarction (0.05 %), arrhythmia, perforation of cardiac chamber or PA, death.
Contrast Agents
Nonionic low-osmolar contrast agents preferred.
Significantly lower osmolality associated with lower risk of adverse reactions (arrhythmia, hypotension, nausea, increased LVEDP, pulmonary edema).
All agents have equal risk of contrast induced nephropathy (CIN).
CIN [7] is defined as rise in creatinine >0.5 mg/dL or 25 % above baseline within 48 h.
Incidence 2 %.
Increased risk in patient with chronic renal insufficiency, diabetes mellitus (DM), anemia and the elderly.
Progression to end stage renal disease is very rare.
Prehydration can reduce the risk of CIN.
Contrast allergy [8]
Severe reactions in ∼1 % of patients.
Prophylaxis: 60 mg prednisone or 100 mg hydrocortisone 12 h and immediately prior to procedure, diphenhydramine 25–50 mg IV immediately prior.
Closure Devices
Four types: suture, collagen plug, passive hemostatic patch, metallic clips.
Allow for sheath removal in anti-coagulated patients and earlier ambulation (1–2 h versus 4–6 h).
No difference in risk of access site complications versus manual compression [9].
Endomyocardial Biopsy
Indications for endomyocardial biopsy [10]:
New onset heart failure (HF) <2 weeks with dilated LV and hemodynamic compromise
New onset HF 2 weeks to 3 months with dilated LV and ventricular arrhythmia or heart block or failure to respond to therapy
Diagnosis of infiltrative cardiomyopathy (e.g. amyloid and sarcoid)
Monitoring of transplanted heart for rejection.
Complications of endomyocardial biopsy:
Cardiac perforation
Ventricular arrhythmia
Heart block
Tricuspid injury (risk reduced with use of longer sheath).
Right Heart Catheterization (RHC)
Indications for RHC:
Cardiogenic shock
Discordant right and left heart failure
Complicated myocardial infarction (MI)
Severe chronic HF requiring supportive therapy
Diagnosis and assessment of pulmonary hypertension
Differentiation of septic vs. cardiogenic shock
Pericardial disease
Diagnosis and assessment of intracardiac shunts
Congenital heart disease
Complications
Infection
Pneumothorax
Arrhythmia
Carotid artery cannulation
Right atrium (RA)/right ventricle (RV)/PA rupture
Pulmonary infarction
Complete heart block (esp. with baseline left bundle branch block).
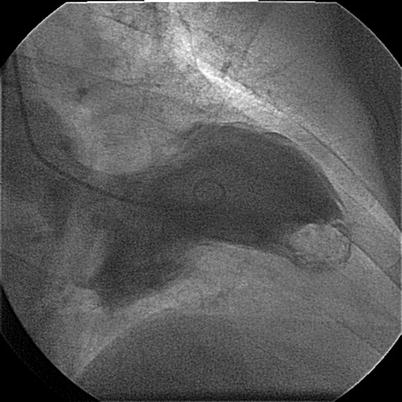
Figure 9-3
A calcified clot in apex in RAO view
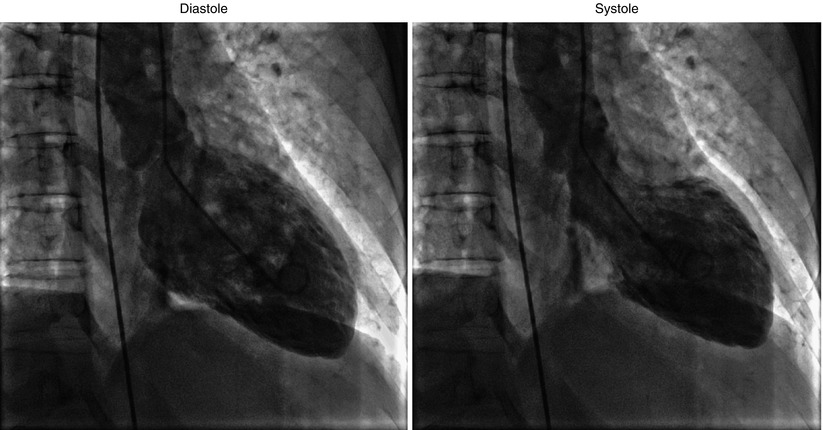
Figure 9-4
Apical ballooning in takotsubo cardiomyopathy (RAO view)
(A)
Pressure measurement (Fig. 9-5):
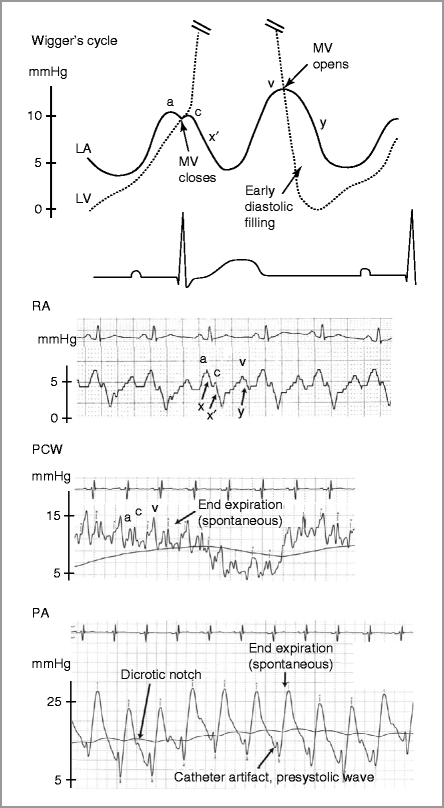

Figure 9-5
Normal right heart catheterization tracings. LA left atrium, LV left ventricle, MV mitral valve, PA pulmonary artery, PCWP pulmonary capillary wedge pressure, RA right atrium
Zero reference pressure is established at the level of the atria
Atrial pressure: three waves
(a) atrial systole
(c) closing of AV valve
(v) RV systole
Two descents
(x) atrial relaxation with open AV valve
(x’) continued atrial relaxation with closed AV valve
(y) tricuspid valve opening
Spontaneous respirations: pressure decreases with inspiration and increases with exhalation
This is reversed with mechanical respiration.
PCWP gives good estimate of mean LA pressure but is delayed and blunted compared to direct LA pressure [11] (Fig. 9-6, Table 9-1)
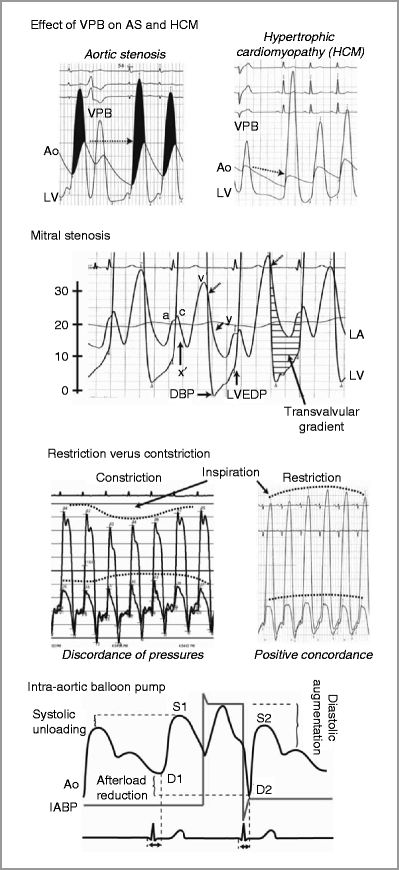
Figure 9-6
Common abnormal pressure tracings. Ao aorta, AS aortic stenosis, DBP diastolic blood pressure, HCM hypertrophic cardiomyopathy, IABP intraaortic baloon pump, LA left atrium, LV left ventricle, LVEDP left ventricular end diastolic pressure, VPB ventricular premature beat
Table 9-1
RHC normal values
Measure
Normal range
Comment
Right atrium
1–6 mmHg
Equivalent to CVP and RVEDP in absence of TV disease
Right ventricle
15–25/1–8 mmHg
Pulmonary artery
15–25/4–12 mmHg
PCWP
4–12 mmHg
Equivalent to LAP and LVEDP in absence of MV disease
Left atrium
2–12 mmHg
Left ventricle
90–140/5–12 mmHg
Cardiac output
4–6 L/min
Cardiac index
2.4–4 L/min/m2
CO/BSA
Systemic vascular resistance (SVR)
700–1,600 dyn × s/cm5
SVR(Woods) = (MAP − RA)/CO
1 Wood = 80 dyn × s/cm5
Pulmonary vascular resistance (PVR)
20–130 dyn × s/cm5
PVR (Woods) = (Mean PA − PCWP)/CO
>240 dyn × s/cm5 → limit heart transplant eligibility
(B)
Get Clinical Tree app for offline access

Cardiac output (CO):
Thermodilution
Tends to underestimate CO with aortic regurgitation (AR), mitral regurgitation (MR) or tricuspid regurgitation (TR)
Inaccurate in low CO state (CO <2.5 L/min), shunt or irregular rhythm.
Fick Method
< div class='tao-gold-member'>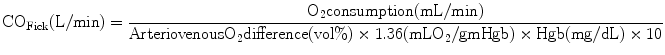 Only gold members can continue reading. Log In or Register to continue
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


