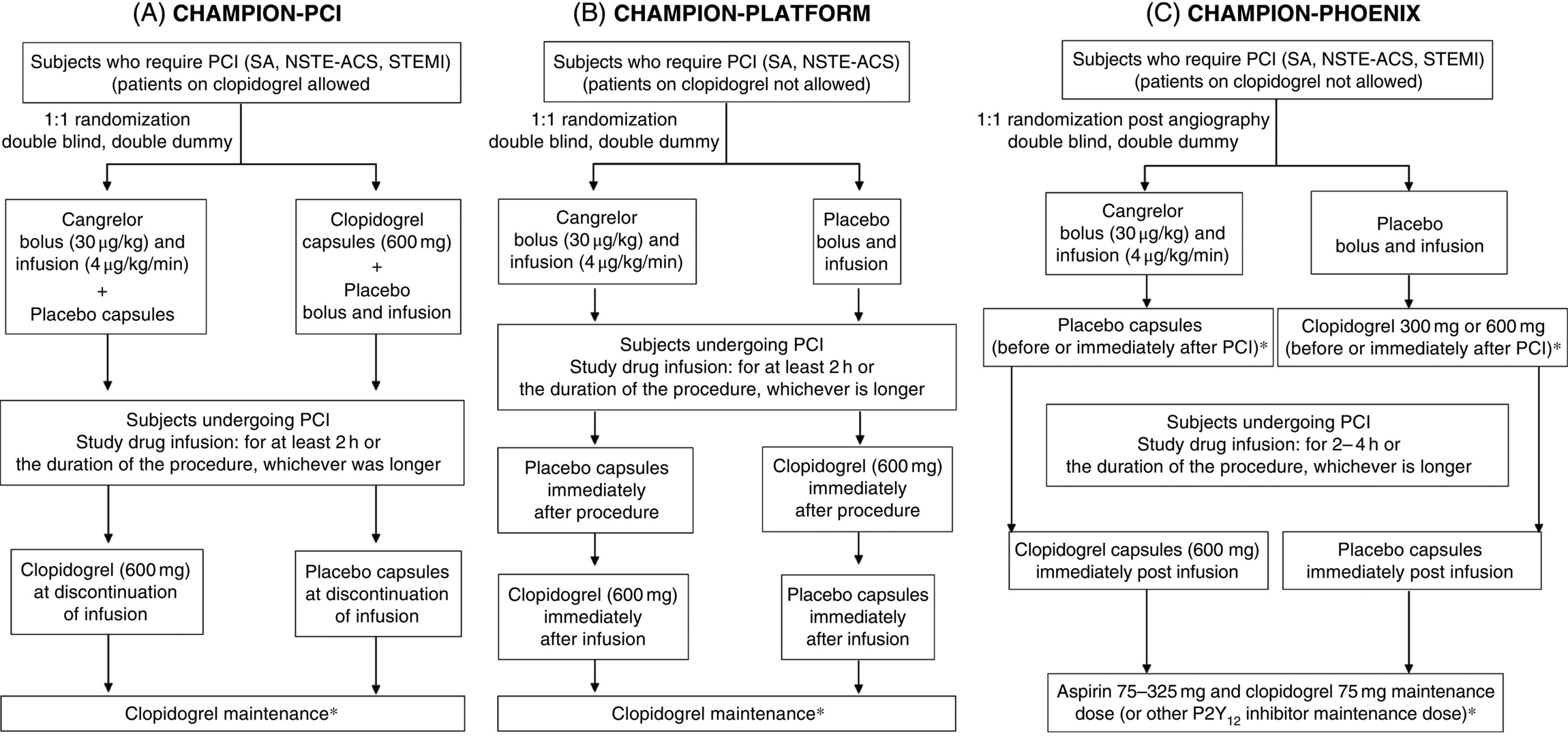
22 Francesco Franchi,Fabiana Rollini, Ana Muñiz-Lozano and Dominick J. Angiolillo University of Florida College of Medicine, Jacksonville, FL, USA Oral adenosine diphosphate (ADP) P2Y12 receptor inhibitors have consistently demonstrated to reduce the risk of thrombotic events in patients with acute coronary syndrome (ACS) and undergoing percutaneous coronary intervention (PCI) [1, 2, 3]. However, oral P2Y12 receptor inhibitors mostly utilized in clinical practice (clopidogrel, prasugrel, and ticagrelor) have several limitations. Clopidogrel is a prodrug characterized by high variability in absorption, delayed onset of action, drug–drug interactions, incomplete P2Y12 receptor inhibition, and broad interindividual response variability [4]. Prasugrel is, like clopidogrel, a thienopyridine but with faster and more potent P2Y12 receptor inhibition [5]. Ticagrelor, a direct-acting reversible P2Y12 inhibitor, also has faster and more potent platelet inhibitory effects compared with clopidogrel [6]. However, both prasugrel and ticagrelor have delayed and suboptimal platelet inhibition in the first 2 h following drug administration in patients with ST-elevation myocardial infarction (STEMI), a setting where immediate and potent platelet inhibitory effects are needed [7, 8]. In addition, clopidogrel and prasugrel are irreversible platelet inhibitors, with duration of effects of 7–10 days [9], while ticagrelor, even if it is a reversible inhibitor, should be stopped at least 5 days before surgery [6]. Therefore, many physicians refrain from administering P2Y12 inhibitors before angiography, since irreversible platelet blockade increases the risk of coronary artery bypass grafting (CABG)-related bleeding [2, 3]. In addition to the concerns raised on the limitations of oral P2Y12 receptor inhibitors, about 5–20% of patients need to undergo some kind of surgery within the year after stent implant or ACS diagnosis. The risk of perioperative cardiac ischemic events, particularly stent thrombosis (ST), is high in these patients, because surgery has a prothrombotic effect and antiplatelet therapy is often withdrawn in order to avoid bleeding [10]. Several antithrombotic approaches have been proposed for bridging strategies. However, heparin and low-molecular-weight heparin are generally not recommended, because they do not have antiplatelet effects (unfractionated heparin can actually increase platelet reactivity). Small-molecule glycoprotein IIb/IIIa inhibitors (GPIs), such as tirofiban and eptifibatide, have been tested in small studies because of their rapid onset of action and consistent platelet inhibitory effects [11]. However, they have a relatively slow offset of action (~4–6 h), and their prolonged administration in the setting of bridging can increase the risk of bleeding [12]. Ultimately, current P2Y12 inhibitors are only available orally and cannot provide reliable inhibition in patients who are unable to swallow or rapidly absorb medications taken orally, such as patients who are sedated, intubated, or in shock or those with nausea or vomiting [13]. Overall, these findings underscore the need for a P2Y12 inhibiting agent available for intravenous use with a prompt and potent onset of action, limited interindividual variability, and fast offset of action. Cangrelor is an intravenous adenosine triphosphate (ATP) analog which directly inhibits, without being metabolized, the P2Y12 receptor in a reversible manner; it has a rapid onset and offset mechanism of action and achieves high degrees of platelet inhibition [14]. This chapter provides an overview of the current status of knowledge on cangrelor, focusing on its pharmacological properties and clinical development. ATP targets mainly the P2Y1 receptor, which is involved in platelet shape change and helps amplify platelet responses mediated by other agonists [15]. It has less affinity to the P2Y1 and P2Y12 receptors because it is rapidly metabolized by ectonucleotidases [16]. The final therapeutic compound of cangrelor (2-trifluoropropylthio, N-(2-(methylthio)ethyl)-β,γ-dichloromethylene ATP) was modified from ATP to confer higher affinity, longer half-life, and higher antagonistic property with a potency increased by six times [14]. These structural changes resulted in unique pharmacological advantages. Cangrelor has high affinity for the P2Y12 receptor, has a higher resistance to ectonucleotidases [16], does not require hepatic conversion, and is directly active. Cangrelor has a linear dose-dependent pharmacokinetic profile, with predictable plasma levels, and reaches steady-state concentrations within minutes, providing stable pharmacodynamic effects. Moreover, it has a very short half-life (2–5 min) leading to a fast offset of action (30–60 min) [17]. Cangrelor inhibited thrombus formation and ADP-induced platelet aggregation in animal models and showed lower increase in bleeding time when compared to GPIs [18]. Infusion in a range of 0.1–4 µg/kg/min showed a dose-dependent effect on platelet inhibition. The steady-state plasmatic concentrations were achieved rapidly and platelet inhibition reversed after 20 min of infusion discontinuation [19]. Greater inhibition of ADP-induced platelet aggregation and less response variability with cangrelor than with clopidogrel were also demonstrated, and cangrelor was able to essentially abolish ADP-induced platelet aggregation in clopidogrel-treated patients [20, 21]. The safety and preliminary efficacy results of cangrelor have been evaluated in several phase II clinical investigations. In ACS patients, cangrelor showed a mean half-life of less than 5 min, a small volume of distribution, and a dose-dependent effect. The steady-state level of inhibition was achieved within 30 min, and the proportions of patients with 100% inhibition of maximal platelet aggregation induced by ADP at 24 h were 77% with 2 µg/kg/min and 86% with 4 µg/kg/min. All patients had greater than 80% inhibition of aggregation, and 60% of the baseline platelet aggregation was recovered within 1 h after stopping infusion. Minor bleedings were observed in 56% patients, with no major bleeding and a poor correlation between bleeding time and drug plasmatic concentrations [22]. Another study on STEMI patients demonstrated no difference between full dose of alteplase and combination of half-dose of alteplase along with cangrelor in Thrombolysis in Myocardial Infarction (TIMI)-3 flow at 60 min, while cangrelor alone was significantly inferior. However, a trend toward improvement in ST-segment recovery was observed in patients receiving combination therapy. Bleeding events and major adverse clinical events were similar among groups [23]. Among patients undergoing PCI, cangrelor demonstrated similar 30-day incidence of cardiac adverse events compared with abciximab (7.6 vs. 5.3%; P = ns). Platelet inhibition was similar during the steady-state phase, but 12–24 h after terminating infusion, it persisted in the abciximab group while returned to baseline in cangrelor-treated patients. No significant difference in rates of bleeding was found comparing cangrelor either with placebo or abciximab [24]. Since diabetes mellitus (DM) is known to be associated with impaired response to antiplatelet therapies [25], the pharmacodynamic effect of cangrelor was compared in clopidogrel-naïve coronary artery disease patients (n = 103) with and without DM. After in vitro incubation with 500 nmol/L of cangrelor, a significant reduction in vasodilator-stimulated phosphoprotein P2Y12 reactivity index (VASP-PRI) was observed in the overall population (80.6 ± 14.0% relative reduction in PRI). Interestingly, this reduction was consistent in DM and non-DM patients (P < 0.0001 for both comparisons), with no difference in PRI values between groups (16.1 ± 12.3 vs. 16.8 ± 11.3; P = 0.346). Moreover, the dose-dependent effect of cangrelor was not influenced by DM status [26]. A study on healthy volunteers randomized to receive clopidogrel (600 mg loading dose) either simultaneously or immediately after cangrelor administration (bolus of 30 µg/kg and 1-h infusion of 4 µg/kg/min) showed a competitive interaction between the two drugs. The very high affinity of cangrelor to P2Y12 receptor prevents the active metabolite of clopidogrel to bind to the receptor during infusion. Therefore, clopidogrel-induced platelet inhibition is precluded if the P2Y12 receptor is already inhibited by cangrelor [27]. The same effect was demonstrated also on prasugrel ability to inhibit platelet aggregation. In contrast, addition of cangrelor after preincubation with active metabolites of clopidogrel or prasugrel led to a sustained platelet inhibition [28]. Instead, no significant pharmacodynamic interaction was observed between cangrelor and the direct-acting P2Y12 receptor antagonist ticagrelor using an ex vivo canine model [29]. Overall, these observations underline the need of an appropriate transitioning strategy from cangrelor to oral P2Y12 receptor inhibitors. Thus far, transitioning studies have only been performed from cangrelor to clopidogrel in which clopidogrel should be administered only after cangrelor infusion discontinuation [30, 31, 32, 33]. Transitioning studies from cangrelor to prasugrel or ticagrelor have yet to be reported. The efficacy of cangrelor in patients undergoing PCI predominantly with ACS was first studied in two parallel large phase III trials, the Platelet Inhibition with Cangrelor in Patients Undergoing PCI (CHAMPION-PCI) trial [30] and the Intravenous Platelet Blockade with Cangrelor during PCI (CHAMPION-PLATFORM) trial [31]. The primary end point for both studies was a composite of death from any cause, myocardial infarction (MI), and ischemia-driven revascularization (IDR) at 48 h post randomization. In CHAMPION-PCI, patients were randomized to receive a 30-µg/kg bolus dose of cangrelor followed by a 4-µg/kg/min infusion (administered within 30 min of the PCI start and continued for a minimum of 2 h and no longer than 4 h) plus 600-mg loading dose of clopidogrel at the end of infusion or a 600-mg loading dose of clopidogrel prior to the start of PCI. In CHAMPION-PLATFORM, patients were randomized to receive cangrelor bolus of 30 µg/kg and infusion 4 µg/kg/min or a matching placebo bolus and infusion. Patients in the placebo group received a loading dose of 600 mg of clopidogrel at the end of the PCI, while patients in the cangrelor group at the end of infusion (Figure 22.1). In both trials, patients randomized to cangrelor received their loading dose of clopidogrel after infusion discontinuation, in order to avoid interaction between clopidogrel and cangrelor. Patients with STEMI and on prior clopidogrel therapy were eligible for CHAMPION-PCI but excluded from CHAMPION-PLATFORM. However, enrollment was terminated early for low likelihood of achieving the primary end point (98% of patients enrolled in CHAMPION-PCI and 84% in CHAMPION-PLATFORM), and both trials failed to demonstrate the superiority of adjunctive cangrelor therapy for the primary end point (7.5% vs. 7.1%; OR 1.05; 95% CI, 0.88–1.24; P = 0.59 and 7% vs. 8%; OR 0.87; 95% CI, 0.71–1.07; P = 0.17, respectively). Of note, a benefit with cangrelor was shown in prespecified secondary end points not dependent on cardiac biomarkers and, overall, in the rate of ST. In addition, the time from randomization to PCI was very short, making difficult the assessment of PCI-related MI. Interestingly, the rate of major bleeding was higher with cangrelor according to Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) criteria but not with other bleeding scores.
Cangrelor
Pharmacological properties
Preclinical studies and early phase clinical investigations
Drug interactions
Cangrelor in patients undergoing PCI: phase III studies

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


