25 Bypass Graft Intervention
 Scope of the Problem
Scope of the Problem
Coronary artery bypass grafting (CABG) is one of the most common surgical procedures. The efficacy of CABG has been enhanced by the use of arterial grafts, off-bypass procedures, and minimally invasive surgical techniques; in addition, attempts have been made to improve graft longevity with antiplatelet agents and lipid-lowering therapy. However, the temporary nature of the palliative effect remains a significant healthcare problem. Severe myocardial ischemic syndromes occur in 3% to 5% of patients immediately after surgery; thereafter, recurrent ischemic symptoms appear in 4% to 8% of the millions of surviving post-CABG patients annually. Saphenous vein graft (SVG) attrition, the most common cause, is up to 30% during the first year and is about 4% per year thereafter. At 10 years, only 40% of patent grafts are free of significant stenosis. At Emory University and the Cleveland Clinic, reoperation was required in about 3% of patients by 5 years, 15% by 10 years, and 30% by 15 years. Even in the most experienced centers, the risk of in-hospital death and nonfatal Q-wave myocardial infarction (MI) is triple that of the initial operation. In New York State, in-hospital mortality was 4.1% for initial operations but 11%, 25%, and 39% for first, second, and third reoperations, respectively.1 In addition to being more risky, reoperative surgery was associated with less complete anginal relief and reduced graft patency. These factors have promoted a conservative approach to reoperation and favored the use of percutaneous coronary intervention (PCI).2–6 In addition, many symptomatic patients who are candidates for percutaneous methods would not be considered for reoperation because of jeopardy to limited myocardium, risk to patent grafts, lack of suitable conduits, poor left ventricular (LV) function, advanced age, or coexisting medical problems. In the past decade at Emory University Hospital, approximately 15% of the patients who underwent PCI had had prior CABG, and bypass graft intervention was the most common indication. It is in this difficult group of patients—those requiring bypass graft intervention—that decision making is particularly critical owing to the increased risk and reduced long-term benefit of bypass graft intervention.
 Indications for Intervention
Indications for Intervention
Patients who experience recurrent ischemia after coronary bypass surgery have diverse anatomical problems; therefore selection for PCI must be based on careful analysis of the probabilities of outcomes compared with competing strategies. The status of the left anterior descending (LAD) coronary artery and its graft significantly influences revascularization choices because of its impact on long-term outcome and the lack of survival benefit of reoperative surgery to treat non-LAD coronary artery-related ischemia.7,8 Factors favoring surgical revascularization include multiple vessel involvement, severe vein graft disease, poor LV function, more total occlusions of native coronary arteries, and the availability of arterial conduits (Table 25-1).8 Because both the choice of percutaneous methods and the relative effectiveness of each are often influenced by the time elapsed since surgery, indications are considered in relation to this factor.
TABLE 25-1 Significant Predictors of Method of Revascularization in Decreasing Order of Importance
Variable Chi-Square Odds Ratio* Confidence Interval (95%)
CABG, coronary artery bypass grafting; EF, ejection fraction; LAD, left anterior descending; LIMA, left internal mammary artery; LMT, left main trunk.
* Odds ratio > 1 denotes a higher likelihood of PCI.
† Odds ratio calculated for 75th vs. 25th percentile for the respective variable in the cohort.
From Brener SJ, Lytle BW, Casserly IP, et al. Predictors of revascularization method and long-term outcome of percutaneous coronary intervention or repeat coronary bypass surgery in patients with multivessel coronary disease and previous coronary bypass surgery. Eur Heart J. 2006;27:413–418.
Early Postoperative Period
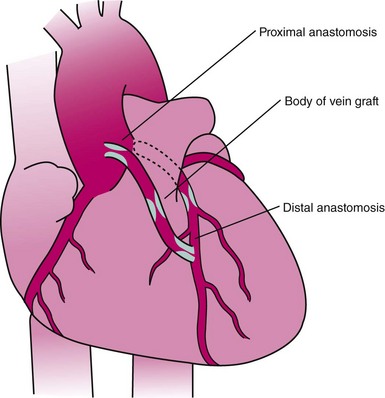
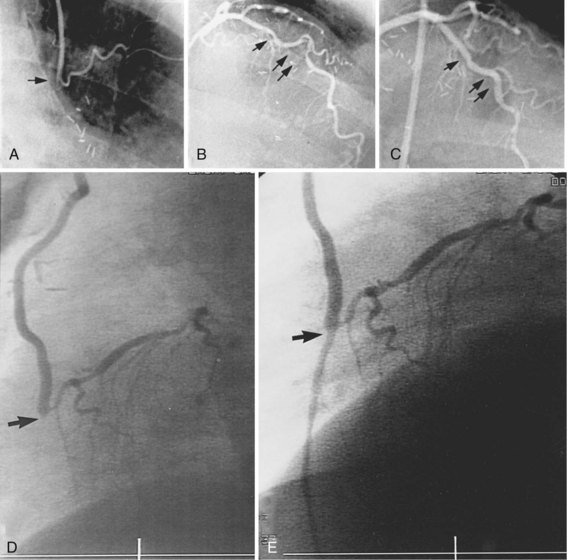
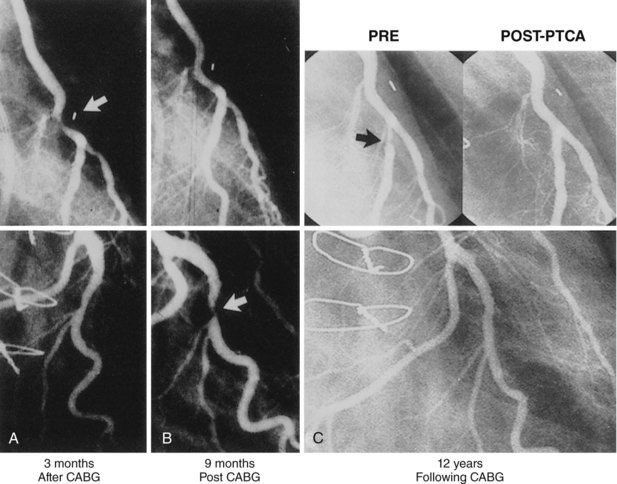
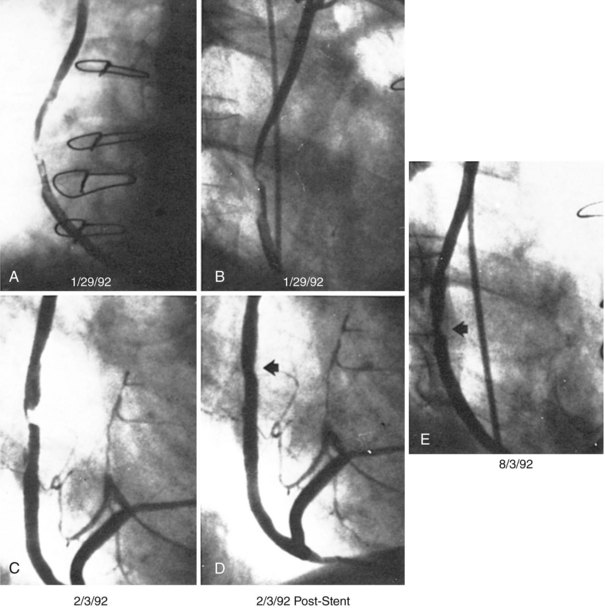
The performance of routine intraoperative angiography at the conclusion of CABG at one center and three recent trials in which routine angiography was performed at 6 to 12 months to assess graft patency have provided sobering insights regarding contemporary CABG.9–12 Among 366 consecutive patients who underwent intraoperative completion angiography, Zhao and colleague found that 12% of bypass grafts had defects important enough to require intervention (open surgical revision 3.4%, open-chest PCI 6%, and minor adjustment 2.8%).9 In a randomized trial of over 3,000 patients, graft failure at 1 year occurred in 30% of saphenous vein grafts (SVGs),10 while SVG failure occurred in 23% performed off pump and 16% on pump in another report,11 and 38% of gastroepiploic artery grafts failed in a third.12 Graft failure was associated with death, new MI, or repeat revascularization in 26% of patients.10 Recurrent ischemia within days of surgery is usually related to acute vein graft thrombosis. However, stenosis may exist at proximal or distal anastomoses (Fig. 25-1); a bypass graft may be kinked; the wrong vessel may have been bypassed; or the revascularization may have been rendered incomplete as a result of diffuse disease, stenoses distal to graft insertion, or inaccessible intramyocardial position of a recipient artery. To determine the cause of severe early postoperative myocardial ischemia and define therapeutic options, coronary arteriography has been carried out within a few hours of surgery in 3% to 4% of patients in some centers,13–15 and this strategy is recommended. PCI in patients with early ischemia after CABG is a class I indication in the American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI) PCI guidelines.16 Although 44 (29%) of 145 patients catheterized because of ischemia early after surgery had no apparent cause for ischemia, most patients had correctable problems; 30 patients had emergency reoperation, and 44 underwent PCI.13–15 Graft occlusion or stenosis was present in about 60% of catheterized patients. In 7 patients, focal stenosis was present in a venous or arterial graft distal anastomosis, and balloon dilation across suture lines was safe in these patients. However, in our experience (Fig. 25-2) and in that of others, extreme care is warranted even a few hours after surgery to ensure an intracoronary position of the steerable guidewire. In addition, balloon sizing should be conservative, because we are aware of unreported cases of suture-line disruption and severe hemorrhagic complications. Immediate access to a covered stent is essential should suture line perforation occur. Patients at increased risk for early postoperative ischemia include those undergoing minimally invasive and “off-bypass” techniques and those receiving non-internal mammary artery (IMA) arterial grafts. When ischemia recurs 1 to 12 months after surgery, perianastomotic stenoses are among the most common problems (Fig. 25-3). Stenotic lesions of the distal anastomosis of saphenous vein or arterial grafts can be successfully dilated with balloon angioplasty at this time with little morbidity and good long-term patency in 80% to 90% of patients.3,17 Stenoses, or in some cases total occlusions, of the middle or distal portions of the IMA or radial artery grafts may be dilated successfully (Fig. 25-4), especially when the presence of a short occlusion can be documented. Stenotic lesions of mid-SVGs occurring within a year of surgery are usually due to intimal hyperplasia; these lesions can be dilated with balloon angioplasty and/or stented with little risk of distal embolization, but recurrence in about 50% of cases in our experience and periprocedural graft perforation has been observed. Stents, directional atherectomy, and excimer laser angioplasty have all been tried for the treatment of proximal anastomotic lesions with excellent initial results but significant rates of restenosis. There are few data regarding use of drug-eluting stents at this site.
More than 3 Years after Surgery
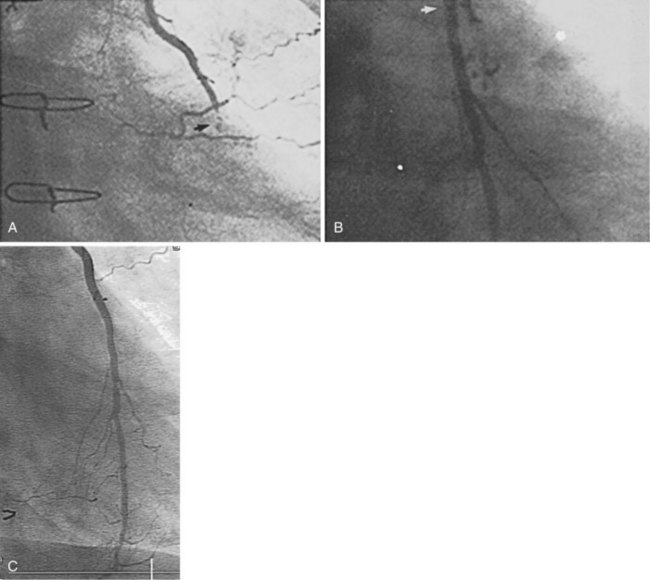
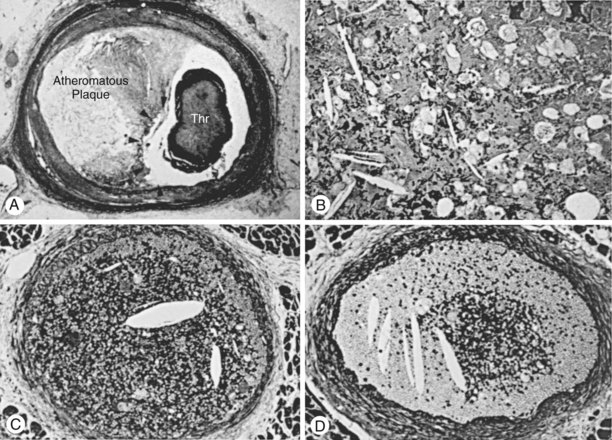
Beginning about 3 years after implantation, atherosclerotic lesions appear in vein grafts with increasing frequency. Unstable ischemic syndromes are common, and aggressive invasive evaluation and therapy are indicated. In about 70% of post-CABG patients presenting with acute coronary syndrome, the culprit lesion is located in a SVG. Atherosclerotic plaques in vein grafts contain foam cells, cholesterol crystals, blood elements, and necrotic debris, with less fibrocollagenous tissue and calcification than is present in native coronary arteries (Fig. 25-5). Consequently the plaques in older vein grafts may be softer and more friable as well as being larger than those observed in native coronary arteries, and they frequently have associated thrombus formation (Fig. 25-6). Atheroembolism related to graft intervention may have catastrophic consequences. Consequently bulky vein graft lesions (those with a large potential atheroma mass) should be avoided if possible and embolic protection utilized if not. Improved initial outcome has been reported with stent placement compared with balloon dilation in SVGs. However, long-term results in SVGs, even with stents, have been disappointing. Data on long-term outcomes with drug-eluting stents in SVGs are limited (see below). The role of percutaneous techniques in totally occluded SVGs is controversial. Balloon angioplasty alone has resulted in high complication rates and low patency in most reports. Unfortunately, prolonged intracoronary thrombolytic therapy has been associated with thromboembolic MI, hemorrhagic complications, and relatively low long-term patency. ACC/AHA/SCAI guidelines consider chronically occluded SVG intervention to be a class III indication (“procedure not useful, may be harmful”).
Acute MI
After coronary bypass surgery, approximately 3% of patients experience acute MI annually. Because these patients were excluded from early reperfusion trials, therapy has been based on clinical experience and remains controversial. Reports from the National Registry of Myocardial Infarction-2 indicate that patients with prior bypass surgery have a high in-hospital mortality with reperfusion strategies, probably attributable to the presence of multivessel disease, prior MI, advanced age, and comorbidity. In 50% to 70% of patients, the culprit vessel has been found to be a vein graft, and considerable lesion-associated thrombus was a common accompaniment. Intravenous thrombolytic therapy is less effective in SVGs.18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree