Chapter 29 Bronchoscopic Treatment of a Large Right Mainstem Bronchial Stump Fistula
Case Description
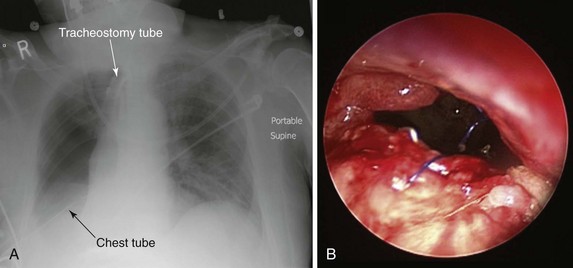
The patient was a 79-year-old male referred for treatment of a large right pneumonectomy stump fistula. He had multiple comorbidities, including severe chronic obstructive lung disease (COPD), systemic hypertension, coronary artery disease, abdominal aortic aneurysm, and carotid artery stenosis, and a remote history of thyroid carcinoma. He had been diagnosed with squamous cell carcinoma 6 months earlier at another institution, where he had undergone right upper lobectomy and radical mediastinal lymph node dissection. Positive surgical margins prompted completion of pneumonectomy several days later. Postoperative hemorrhage required repeat thoracotomy within hours after the pneumonectomy had been performed. The postoperative course was complicated by prolonged mechanical ventilation for respiratory failure, pulmonary embolism, residual left femoral deep vein thrombosis warranting anticoagulation, and placement of an inferior vena cava filter. A tracheotomy was performed on postoperative day 7. Three weeks after surgery, the patient had developed increasing-right sided pleural effusion, copious secretions, and fever. Flexible bronchoscopy revealed a post pneumonectomy stump fistula. A 32-French chest tube was placed into the right hemithorax, and this revealed the presence of a large air leak. Sputum cultures were positive for Pseudomonas aeruginosa, and the patient was started odn intravenous imipenem and amikacin. He was considered inoperable and was referred to our institution for possible bronchoscopic treatment of his right post pneumonectomy stump fistula. Chest radiograph at admission showed an air-fluid level in the right hemithorax consistent with bronchopleural fistula (BPF). Bronchoscopy showed a large BPF due to dehiscence of the right mainstem bronchial stump (Figure 29-1).
Discussion Points
1. List four risk factors associated with the development of bronchial stump fistula.
2. List three bronchoscopic treatment modalities for patients with large bronchial stump fistulas.
3. Describe and justify the use of three ventilator management strategies in patients with stump fistula and respiratory failure.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient had a central bronchopleural fistula (BPF).* Bronchial stump dehiscence (failure) represents BPF when disruption of a bronchial closure occurs after anatomic pulmonary resection. Early failure (within a few days to a few weeks following surgery) is usually the result of a technical problem such as a stapler misfiring, loose or broken sutures, or excessive tension with stump closure, causing poor apposition of tissues. Following pneumonectomy, bronchial stump dehiscence within the first few days to few weeks typically presents with a large air leak and coughing of copious quantities of secretions. Respiratory distress may occur secondary to spillage of pleural fluid through the bronchial stump into the contralateral lung or by loss of air into the empty pleural space, leading in some cases to a tension pneumothorax. Late stump failure (occurring later than 2 to 4 weeks after surgery but usually within 90 days of operation) is typically secondary to weak bronchial tissue and infection (bronchitis, empyema), which lead to loss of integrity of the bronchial stump.1 Patients present with cough and expectoration of frothy, blood-tinged secretions. Fever is often present, and patients may have sweats and chills secondary to infection within the pleural space.
BPFs after pneumonectomy, as seen in this case, are not uncommon2,3 and are associated with high mortality rates, usually resulting from empyema.4 The estimated incidence of post pneumonectomy BPF ranges between 3% and 28%. Although the perioperative mortality of pneumonectomy overall is now less than 7%, this rises to 25% when complicated by empyema, and it may be as high as 50% when associated with fistula.*3,5,6 In addition to empyema, the main complication of BPF is aspiration pneumonia caused by spillage of contaminated secretions into the contralateral healthy lung, potentially leading to adult respiratory distress syndrome (ARDS). The resulting impaired respiratory mechanics, contralateral lung contamination, and chronic pleural sepsis contribute to poor outcome.7
The bronchial stump after pneumonectomy or lobectomy could be fragile in cases of bronchial stump ischemia, extensive peribronchial dissection, inflammation at the suture line, or residual tumor at the bronchial stump margins. Several other risk factors also predispose to fistula formation and bronchial stump dehiscence, including preoperative chemotherapy or radiation therapy, right pneumonectomy, male gender, a large and long stump, postoperative mechanical ventilation, tracheostomy, and excessive dissection and tension at the bronchial suture line. Right-sided pneumonectomy, as performed in our case, is considered a major risk factor for stump disruption, in part because of the larger size and greater tendency of the right main bronchus to spring open, especially in the setting of positive-pressure ventilation, and because surgery-related alterations in the right bronchial microvasculature may lead to stump necrosis.3 BPF after right pneumonectomy occurs with significantly increased frequency compared with left pneumonectomy (13.2% vs. 5.0%).8 Pathophysiologically, this may be explained by at least two factors: First, the most common vascular supply to the right mainstem bronchus consists of a single bronchial artery, whereas the left mainstem bronchus is most commonly supplied by two bronchial arteries. Second, the left mainstem bronchus is protected beneath the aortic arch and surrounding vascularized mediastinal tissue. Another possible risk factor, present in our patient, is the use of sutures rather than staples for stump closure (Figure 29-1). In this regard, in a series of 713 pneumonectomies, Deschamps and colleagues observed an increased frequency of post pneumonectomy BPF following suture closure versus stapled closure of the bronchus (incidence of 12.5% vs. 3.8%, respectively).4
As part of the initial evaluation, chest computed tomography (CT) may reveal a direct sign of BPF: A fistulous tract is noted between the bronchus or lung and the pleural space. Indirect signs include air bubbles beneath the bronchial stump and “suspected fistula” defined as a suspicious but not definite communication between the pleural space and the airway or lung parenchyma.9 CT is useful not only for visualization and localization of BPF but also for identification of the cause, number, and size of the BPF and of underlying lung lesions (e.g., post lung resection, necrotizing pneumonia).9
Diagnosis on bronchoscopy is made by observing the direct signs of BPF. These are defined by the presence of a fistula opening at the bronchial stump or by the appearance of dye, previously injected into the airways during bronchoscopy, in the chest tube collection device. Air bubbling during the respiratory cycle (see video on ExpertConsult.com) (![]() Video VI.29.1) and purulent secretions from the segmental airways are indirect signs of peripheral BPF. As part of an initial evaluation, even asymptomatic patients with clinically or radiographically suspected BPF should undergo flexible bronchoscopy for evaluation of the stump.* A fistula will often be visible, and a small amount of air bubbling may be observed with careful inspection of the stump (see video on ExpertConsult.com) (
Video VI.29.1) and purulent secretions from the segmental airways are indirect signs of peripheral BPF. As part of an initial evaluation, even asymptomatic patients with clinically or radiographically suspected BPF should undergo flexible bronchoscopy for evaluation of the stump.* A fistula will often be visible, and a small amount of air bubbling may be observed with careful inspection of the stump (see video on ExpertConsult.com) (![]() Video VI.29.1).† In our patient, bronchoscopy revealed a large BPF due to complete stump dehiscence (see Figure 29-1).
Video VI.29.1).† In our patient, bronchoscopy revealed a large BPF due to complete stump dehiscence (see Figure 29-1).
Patient Preferences and Expectations
Because the patient’s tumor was now completely resected, the wife expressed her wish to be aggressive and “do everything possible” to restore her husband’s health. We did not consider her requests unrealistic. In today’s health care environment, physicians’ relationships with both patients and society have undergone important changes. For instance, patients are increasingly viewed as active “consumers” able to demand and are often encouraged to command and expect enhanced services, including extended hours and rapid access. In opposition to some examples in the managed care environment, where patients and health care providers might feel they are the victims of decreased access or have difficulty obtaining second opinions or referrals to specialists outside of their plans, the easy availability of health information coupled with a sense of entitlement is causing a power shift in the physician-patient relationship whereby patients might, on the basis of unrealistic expectations, impose their desire for costly tests and procedures in their attempt to do everything possible to reverse the effects of their illness, even if such tests or procedures are not believed to be medically indicated.10
Procedural Strategies
Indications
There was little doubt that without treatment of the BPF, this patient would have continued to deteriorate and eventually would have died from empyema and pneumonia in the contralateral lung. Given the reasonable reluctance to re-intervene surgically, a bronchoscopic attempt at closure of the fistula was warranted. Management strategies for BPF depend on a number of factors, including underlying cause, size, time of onset of the fistula post surgery, and health status of the patient. Surgery is the treatment of choice for this condition, but bronchoscopic techniques have been advocated as an option when surgery is not possible or has to be postponed.7 Management of patients who have developed BPF and require mechanical ventilation is even more complex. Surgical repair is not a good option for these patients because postoperative mechanical ventilation is associated with a high failure rate owing to persistent barotrauma on the repaired stump.7
In general, nonsurgical, bronchoscopic strategies are categorized as follows: (1) occlusion with indwelling airway stents, (2) occlusion with glue or other materials, and (3) procedures that induce scar tissue formation at the fistula site. The goal of the stent, as chosen in this case, is to provide a tight seal in the airway to prevent spillage of infected pleural fluid into the contralateral lung. Because many types of stents with different biomechanical properties are available, selection of a stent requires consideration of the physical characteristics of the stent and potential associated short-term and long-term complications.11 Several case reports of endobronchial stent insertion for isolated fistulas have been published, but case series of more than two patients are few.12 Obviously, the effect of case selection is difficult to quantify from these publications. However, the feasibility of the technique in selected patients is encouraging. In one study, for instance, authors used specially designed covered metal stents (with a blind ending arm to fit the bronchial stump) in six patients. Patients underwent follow-up CT scan and bronchoscopy to confirm satisfactory stent placement. The mortality rate (mean follow-up, 316 days) was 0% with 67% of patients having the fistula closed; this led to resolution of the empyema.12
Contraindications
No absolute contraindications to rigid bronchoscopy were noted, but general anesthesia was obviously risky in this patient with sepsis from empyema, pulmonary embolism, and multiple cardiovascular problems. In this regard, the physical status classification system of the American Society of Anesthesiologists (ASA) is a relatively simple system that has proved effective in stratifying overall preoperative risks of morbidity and mortality for patients undergoing anesthesia and surgery.13 Complications are much more likely to occur in patients with preexisting disease states. For example, an ASA class 1 patient has no underlying conditions or limitations, but a class 5 patient is not expected to survive the next 24 hours without the proposed intervention. Risks inherent to a specific procedure are not incorporated into the ASA class. The relative risk of serious perioperative complications is 4.4 for ASA patient status 4, as was assigned to ours (i.e., a severe incapacitating disease process that is a constant threat to life), illustrating that increasing serious comorbidities reflected in the ASA classification increase perioperative morbidity.14 Our patient was considered nonoperable because of his poor clinical status and high operative risk. Surgical contraindication was determined by consensus at our multidisciplinary meeting, which included critical care physicians, the interventional pulmonologist, and the consulting thoracic surgeon. The choice of using stent insertion, rather than repeat surgical repair, was thus based on the patient’s unstable respiratory status, poor general health status, and inflamed appearance of BPF margins (see Figure 29-1).
Expected Results
Most published experience related to airway fistulas describes occlusion of tracheal or bronchoesophageal fistulas,15,16 as well as case reports, small case series, and a few review articles pertaining to postsurgical bronchial stump fistulas.17–21 In addition to their described use in patients with an inoperable or unresectable tumor involving the mainstem bronchi and lower trachea, Y stents have been used successfully in patients with trachea-broncho-esophageal fistulas, resulting in improved dyspnea and quality of life and a reduced rate of recurrent infection.22 A Y stent has been reported to successfully cover a BPF in a patient who underwent right upper lobectomy for lung cancer.23 However, the Y stent has patent distal bronchial limbs and thus cannot completely occlude a major post pneumonectomy stump dehiscence. For this purpose, modified versions of the Y stent have involved occlusion of one of the bronchial limbs to occlude or cover the airway defect in patients who are not surgical candidates. In one report, the authors shortened the right limb of a studded Dumon Y stent (Novatech, Cedex, France) and closed the right distal bronchial limb with silicone material cut out from the stent itself.17 This resulted in successful occlusion of a 2 mm bronchial stump fistula. Another modified version of the Y stent is obtained by requesting the manufacturer to customize the stent at the time of manufacture by shortening the right bronchial limb and sealing its distal aspect. Such a stent was used successfully in a patient with a major right bronchial stump fistula and respiratory failure.21 The fact that such a customized stent must be ordered and manufactured, however, may limit wider applicability and may delay scheduling of the therapeutic intervention.
Therapeutic Alternatives for Sealing Off the Fistula
Surgical treatment options include bronchial stump revision with omental or muscular flap coverage. Surgeons usually advocate immediate thoracoplasty and open window thoracostomy when a major bronchial stump dehiscence is suspected, to prevent empyema and aspiration pneumonia on the contralateral side.24,25 At the time of our evaluation, the patient was not considered to be a surgical candidate.
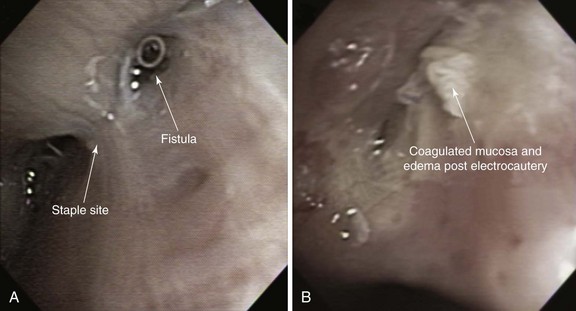
Minimally invasive techniques such as thoracoscopic and bronchoscopic procedures using different glues, coils, and sealants have been performed to attempt closure of BPFs in patients who are deemed to be poor candidates for repeat thoracotomy. Successful bronchoscopic closure of fistulas has been described with the use of ethanol, polyethylene glycol–based gel, fibrin, acrylic glue, cellulose, and gel foam, and placement of angiographic occlusion metallic coils, polidocanol, decalcified spongy calf bone, or unidirectional endobronchial valves or spigots.7 Scar-forming techniques have been reported, in which thermal energy was provided by neodymium-doped yttrium aluminum garnet (Nd:YAG) laser or electrocautery (Figure 29-2). In the absence of tumor or infection, the laser beam or electrocautery probe is directed toward the mucosa surrounding the fistula to induce tissue edema, protein denaturation, and inflammation, eventually facilitating closure of the fistula by fibrosis.26 Scar tissue and BPF closure can also be achieved by submucosal injection of the vein sclerosant polidocanol.27 According to published studies, resolution of BPF using these bronchoscopic techniques ranges from 33% to 58%. These techniques involve repeated bronchoscopic interventions in most patients.26,28,29 Furthermore, they are usually reserved for patients with small fistulas measuring less than 5 mm and for those with fistulas that occur in more distal bronchial regions. The absence of evidence supporting the use of one technique versus another justifies a multidisciplinary approach in which interventional bronchoscopists and thoracic surgeons choose a specific product or technique based on their experience and product availability.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree