Chapter 45 Bronchiectasis
Pathology
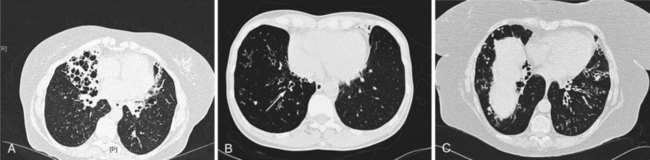
Whitwell classified bronchiectasis into three groups based on pathologic findings—saccular, atelectatic, and follicular—and although these terms may have changed, the descriptions remain the same. Cylindrical bronchiectasis is characterized by bronchi showing a regular outline, with dilated airways only and usually ending abruptly. Varicose bronchiectasis has similarities to the appearance of varicose veins, with dilation that is deformed by areas of relative constriction. These bronchi also have a distorted and bulbous end. Cystic (saccular) bronchiectasis is considered the most severe form of bronchiectasis; its most prominent feature is progressively increasing dilation as the bronchi progress toward the lung periphery and airways, ending in cystlike clusters. These three basic forms of bronchiectasis are demonstrated in Figure 45-1 by high-resolution computed tomography (HRCT) scanning.
Pathogenesis
The cause of non-CF bronchiectasis may only be identifiable in up to 50% of patients. However, a number of recognized conditions and factors are associated with bronchiectasis, and an underlying cause should be assessed in all patients (Box 45-1).
Box 45-1
Underlying Causes of Bronchiectasis
The genetic influence on the development of non-CF bronchiectasis is the subject of ongoing research, often on the role of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The most common mutation of this gene, ΔF508 (F508del), is associated with severe CF (see Chapter 44). Numerous other mutations have been identified and associated with a milder clinical phenotype. As a consequence, late first presentation of CF has been described in patients who would otherwise have been considered to have idiopathic non-CF bronchiectasis. It is now recognized that mutations of the CFTR gene are more frequently observed in patients with bronchiectasis and a normal sweat chloride test than in the general population. Studies show that the spectrum of CFTR genotypes is associated with a continuum of CFTR dysfunction in the airways. Phenotypes range from patients with bronchiectasis, normal sweat test, and no other features suggestive of CF to those with classic CF. Also, some evidence suggests the CFTR mutations are associated with non-CF bronchiectasis and rheumatoid arthritis. Therefore, CFTR dysfunction can be identified as a cause of bronchiectasis in patients previously diagnosed with idiopathic non-CF bronchiectasis, but without fulfilling the diagnostic criteria for CF. How this affects patient management is currently unknown.
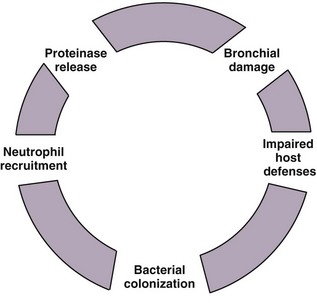
The “vicious cycle” hypothesis of bronchiectasis suggests an initial failure or overwhelmed host defenses, leading to a host-mediated chronic inflammatory response, which in turn causes new or further impairment of mucociliary clearance and defenses, amplifying the problem. This interaction between chronic infection and excessive inflammation, which is predominantly neutrophilic, ensures ongoing damage to the airways and the development and maintenance of the features seen in bronchiectasis. Inflammatory mediators such as the neutrophil chemoattractant interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF-α) are found in bronchial mucosal biopsies and secretions from bronchiectatic airways, in addition to tissue neutrophilia. This initiation of the inflammatory reaction results in the recruitment of phagocytes, dendritic cells, and lymphocytes, which contribute to the adaptive response (Figure 45-2).
Diagnosis and patient assessment
Blood Tests
Inflammatory markers include white blood cell (WBC) count, neutrophil count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) and can be used as nonspecific tests of disease activity and exacerbation severity. These tests can also be used to guide the need for and response to antibiotics in the absence of changes in clinical state or symptomatology of bronchiectatic patients (Box 45-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree