Brain Injury and Neuroprotective Strategies in Pediatric Cardiac Surgery
Christopher E. Mascio
J. William Gaynor
INTRODUCTION
Brain injury is the most common and potentially disabling complication following congenital heart surgery. With improved survival, the focus has shifted to optimizing functional outcomes. An important goal of therapy for every congenital heart surgical patient is to reduce the risk of brain injury as much as possible. Along with updated perfusion, anesthetic, and surgical strategies, techniques for neuromonitoring have been refined and adopted by many centers performing pediatric cardiothoracic surgery. We review neurodevelopmental outcomes, intraoperative neuromonitoring, and current published data concerning neuromonitoring.
NEURODEVELOPMENTAL OUTCOMES
Survival after congenital heart surgery has improved, and the focus has shifted to also optimizing neurodevelopmental outcomes. Survivors of repair of congenital heart disease (CHD) in the neonatal period demonstrate cognitive, motor, speech, visual, and learning abnormalities (1). Determining causation in abnormal neurodevelopment and the occurrence of neurodevelopmental disability is a challenging endeavor. There are both nonmodifiable and modifiable factors associated with adverse neurodevelopmental outcomes (Table 30.1). Genetic predisposition and many other nonmodifiable patient factors, including prematurity, socioeconomic status, and maternal education, have been shown to be risk factors for worse neurodevelopmental outcomes. Additionally, there is increasing evidence that CHD is associated with altered fetal brain growth and development. Modifiable perioperative management factors have also been implicated in altering neurodevelopmental outcome. Over the last few decades, there have been refinements in perioperative care, anesthetic management, and surgical techniques.
Nonmodifiable Factors
Fetal Brain Development
Beginning in the third trimester of fetal life, patients with CHD are known to have smaller gestational age- and weight-adjusted brain volumes with impaired neuroaxonal development and metabolism (2). These abnormalities are most pronounced with more complex types of heart defects, including hypoplastic left heart syndrome (HLHS) and transposition of the great arteries (TGA).
TABLE 30.1. Factors associated with adverse neurodevelopmental outcomes | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Preoperative Brain Abnormalities
Structural malformations of the brain occur at a much higher rate than in the general population (3). Microcephaly, microencephaly, and other malformations, including agenesis of the corpus callosum and an immature cortical mantle, have been documented in necropsy series of patients with HLHS (4). Neonates with CHD can have altered cerebral hemodynamics, often with lower-than-normal cerebral blood flow (CBF) and/or oxygen delivery. One study of neonates with complex CHD used preoperative pulsed arterial spin-label perfusion magnetic resonance imaging (MRI) to quantitate CBF (5). More than half of the cohort had developmental or acquired lesions, and the CBF was less than half of that reported in
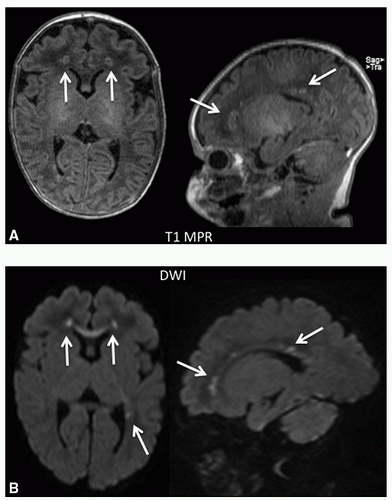
normal, term neonates (6). There was evidence of delayed white matter development, which may lead to an increased risk of white matter injury (WMI), typically periventricular leukomalacia (PVL), and microcephaly. The same study also examined cerebrovascular responsiveness to CO2, and found that PVL was associated with decreased CO2 responsiveness. Abnormal cerebrovascular reactivity to CO2 has been associated with increased mortality and worse neurodevelopmental outcomes (7,8). WMI characterized by PVL is the most common pattern of injury (9). The mechanism of this injury is thought to be due to the effects of hypoxia and/or ischemia on vulnerable premyelinating oligodendrocyte precursors during their most vulnerable time of 24 to 34 weeks’ gestation (10). Up to 40% of neonates with CHD have PVL on preoperative MRI, and PVL has been shown to be associated with poor neurodevelopmental outcomes (5,11,12) (Fig. 30.1). The incidence of PVL is highest in neonates undergoing cardiopulmonary bypass (CPB). A study by Galli et al. (13) found a 54% incidence of PVL in neonates compared to 4% in infants. PVL is the neurologic lesion associated with cerebral palsy in infants born prematurely (14). This pattern of brain injury is seen not only in preterm newborns but also in term neonates with CHD (15). A comparison of term newborns with CHD to a control cohort without heart defects revealed that almost one-third of those with CHD had WMI, while no WMI was seen in those without heart defects (16).
normal, term neonates (6). There was evidence of delayed white matter development, which may lead to an increased risk of white matter injury (WMI), typically periventricular leukomalacia (PVL), and microcephaly. The same study also examined cerebrovascular responsiveness to CO2, and found that PVL was associated with decreased CO2 responsiveness. Abnormal cerebrovascular reactivity to CO2 has been associated with increased mortality and worse neurodevelopmental outcomes (7,8). WMI characterized by PVL is the most common pattern of injury (9). The mechanism of this injury is thought to be due to the effects of hypoxia and/or ischemia on vulnerable premyelinating oligodendrocyte precursors during their most vulnerable time of 24 to 34 weeks’ gestation (10). Up to 40% of neonates with CHD have PVL on preoperative MRI, and PVL has been shown to be associated with poor neurodevelopmental outcomes (5,11,12) (Fig. 30.1). The incidence of PVL is highest in neonates undergoing cardiopulmonary bypass (CPB). A study by Galli et al. (13) found a 54% incidence of PVL in neonates compared to 4% in infants. PVL is the neurologic lesion associated with cerebral palsy in infants born prematurely (14). This pattern of brain injury is seen not only in preterm newborns but also in term neonates with CHD (15). A comparison of term newborns with CHD to a control cohort without heart defects revealed that almost one-third of those with CHD had WMI, while no WMI was seen in those without heart defects (16).
Prematurity
Preterm birth, even in infants without CHD, is a powerful predictor of worse neurodevelopmental outcome. Along with low birth weight, prematurity has been associated with longterm behavioral and learning issues. In a study of 125 very low birth weight preterm infants who were evaluated with the Bayley Scales of Infant and Toddler Development III (BSID-III) at 24 months, later gestational age was associated with better neurodevelopmental outcome (17).
Socioeconomic Factors
Other nonmodifiable patient factors that adversely affect neurodevelopmental outcomes are socioeconomic status and maternal education. Follow-up of the Boston Circulatory Arrest Study (BCAS) cohort at 16 years of age found that family social class and parental IQ were significant predictors of neurodevelopmental outcome (18). In a study of neurodevelopmental outcome after repair of total anomalous pulmonary venous connection, lower socioeconomic status was predictive of lower scores on the Mental Developmental Index (MDI) of the Bayley Scales of Infant Development II (BSID-II) (19). The Single Ventricle Reconstruction trial is the randomized, prospective trial comparing shunt types in the Norwood procedure (20). Evaluation of this cohort at 14 months demonstrated that lower maternal education, in addition to the presence of genetic syndromes or other anomalies and lower birth weight, was associated with lower BSID-II MDI scores (21).
Genetic Syndromes and Polymorphisms
There are many genetic syndromes associated with CHD, and include Down syndrome, Noonan syndrome, Williams syndrome, and DiGeorge syndrome (22q11.2 microdeletion) (22,23,24,25). These syndromes are associated with developmental delay and assigning causation of neurodevelopmental delay can be challenging. For example, those with DiGeorge syndrome have a mean IQ in the 70s, a predisposition to psychiatric disorders, and an increased incidence of white matter abnormalities (26,27,28).
There is also evidence that genetic variants which modify the brain’s response to injury and subsequent recovery may also be important determinants of neurodevelopmental outcomes. The first genetic polymorphism studied in relation to CHD and surgery was apolipoprotein E (APOE) (1). APOE regulates cholesterol metabolism and is the primary lipid transport vehicle in the central nervous system. There is evidence that APOE is important for neuronal repair. There are three APOE alleles (ε2, ε3, ε4) on chromosome 19 that vary by a single amino acid. Investigators at the Children’s Hospital of Philadelphia evaluated the association of APOE genotype and postoperative neurodevelopmental outcome at age 1 year
(1). On the BSID-II, the APOE ε2 allele was associated with a significantly lower Psychomotor Developmental Index (PDI) score after adjustment for perioperative covariates including gestational age, age at operation, sex, race, socioeconomic status, cardiac defect, and the use of circulatory arrest. Further analysis of this group revealed that patient-specific factors, namely the presence of a genetic syndrome, low birth weight, and the APOE ε2 allele, significantly predicted neurodevelopmental outcomes at age 1 year (29). Neurobehavioral outcomes evaluated in the cohort between 4 and 5 years of age revealed that those with the APOE ε2 allele had increased behavioral problems, restricted behavior patterns, and impaired social skills (30). The entire cohort had a significantly higher proportion of patients considered either at-risk or in the clinically significant range for neurodevelopmental problems. Further evaluation of the cohort by a genome-wide association study with adjustment for the effects of APOE identified single nucleotide polymorphisms (SNP) as being associated with neurobehavioral abnormalities (31). Ten SNPs reached a threshold for suggested significant associations with neurobehavioral phenotypes.
(1). On the BSID-II, the APOE ε2 allele was associated with a significantly lower Psychomotor Developmental Index (PDI) score after adjustment for perioperative covariates including gestational age, age at operation, sex, race, socioeconomic status, cardiac defect, and the use of circulatory arrest. Further analysis of this group revealed that patient-specific factors, namely the presence of a genetic syndrome, low birth weight, and the APOE ε2 allele, significantly predicted neurodevelopmental outcomes at age 1 year (29). Neurobehavioral outcomes evaluated in the cohort between 4 and 5 years of age revealed that those with the APOE ε2 allele had increased behavioral problems, restricted behavior patterns, and impaired social skills (30). The entire cohort had a significantly higher proportion of patients considered either at-risk or in the clinically significant range for neurodevelopmental problems. Further evaluation of the cohort by a genome-wide association study with adjustment for the effects of APOE identified single nucleotide polymorphisms (SNP) as being associated with neurobehavioral abnormalities (31). Ten SNPs reached a threshold for suggested significant associations with neurobehavioral phenotypes.
Other Patient Factors
A study comparing the relative contribution of other patient factors (gestational age, genetic syndrome, birth weight, gender, etc.) to management factors during neonatal and infant cardiac surgery on neurodevelopmental outcomes at 1 year of age showed that patient factors explained more of the variability in the PDI (21% vs. 8%) and MDI (13% vs. 5%) scores on the BSID-II than management factors (29). Accordingly, factors such as gender, birth weight, and presence of a genetic syndrome had a more significant impact on neurodevelopmental outcomes than CPB time, circulatory arrest time, and hematocrit. Not all patients with CHD enter the operating room with the same neurodevelopmental prognosis. Interindividual variation of many nonmodifiable patient factors significantly contributes to widely disparate neurodevelopmental outcomes of different patients with the same cardiac diagnosis.
Modifiable Factors
Many studies have examined the impact of different management strategies before and during congenital heart operations on neurodevelopmental outcomes. Studies have found that both preoperative and postoperative management can have a profound impact on neurologic outcome.
Preoperative and Postoperative Management Factors
Hypoxemia and Acidosis
Ductal-dependent cardiac lesions put patients at risk of hypoxemia, acidosis, and cardiovascular collapse if the diagnosis is not known and ductal closure occurs (4,32). Prenatal diagnosis of congenital heart lesions has increased in recent years. This often results in delivery in centers equipped to care for newborns with severe forms of CHD, thereby permitting immediate or early infusion of prostaglandin to maintain ductal patency (33,34,35). Preoperative hypoxemia has been shown to be associated with abnormal neurodevelopmental outcomes. Neurodevelopmental testing on a cohort of patients 5 to 10 years after repair of tetralogy of Fallot or ventricular septal defect in infancy demonstrated more speech and language dysfunction in the group with preoperative hypoxemia and cyanosis (36).
Postoperative systemic oxygen delivery can affect neurodevelopmental outcomes. Prolonged low postoperative cerebral oxygen saturation (<45% for >180 minutes) following the Norwood operation for HLHS was associated with the development of new or worsened ischemia on postoperative brain MRI (37). In a small study of neonates with HLHS who underwent the Norwood procedure, low systemic venous oxygen saturation and hypotension was associated with adverse neurodevelopmental outcomes (38). A study of neonates and infants undergoing biventricular repair without aortic arch reconstruction found that lower Sto2 in the postbypass and early postoperative period was associated with lower PDI scores (BSID-II) and brain hemosiderin on MRI (39).
Hypotension and Cardiac Arrest
In neonates undergoing congenital heart surgery, new postoperative WMI was found to be specifically associated with low mean blood pressure during the first postoperative day (40).
Cardiac arrest places all organs at risk until circulation, either spontaneous or mechanical, is restored. A review of pediatric cardiac extracorporeal membrane oxygenation (ECMO) and extracorporeal cardiopulmonary resuscitation (ECPR) in children with cardiac disease identified 10 studies that examined survival and neurologic outcomes after ECPR (41). Overall survival of ECPR was 49% (range 33%-79%). In the nine studies describing Pediatric Cerebral Performance Category (PCPC) score (an early test of neurologic function), 79% of the survivors had a PCPC score of ≤2, indicating normal or mild neurologic impairment. There is no data describing long-term neurodevelopmental outcomes after cardiac arrest and ECPR.
Intraoperative Management Factors
Temperature
Hypothermia was first used in congenital heart surgery in 1953 to aid in atrial septal defect closure (42). As discussed in detail in Chapter 8, the rationale for hypothermia is protection against ischemic damage by decreasing the metabolic rate. Different levels of hypothermia may be used: moderate (25°C-32°C), deep (18°C-20°C), and deep hypothermic circulatory arrest (DHCA) (16°C-18°C). In the pediatric population, the Q10 (ratio of metabolic rate at two temperatures separated by 10°C) is higher (3.65) than that in adults (2.4-2.8) (43). Hypothermia permits reduced CPB flow rates (or zero flow if employing DHCA) and provides a margin of safety should a disastrous event occur during CPB (44). Hypothermia also reduces the
inflammatory response to CPB and cools tissues that are in contact with the myocardium (45).
inflammatory response to CPB and cools tissues that are in contact with the myocardium (45).
Hypothermia is neuroprotective and the BCAS suggested that a duration of DHCA ≤41 minutes (95% 1-sided lower confidence limit of 32 minutes) is safe (46,47). It should be noted that the equipment, blood gas management (a-stat), and degree of hemodilution (hematocrit of 20%) used in the BCAS are outdated and have been modified (pH-stat, hematocrit no lower than 25%-30%) at most centers.
The frequency of use of hypothermia is surgeon-specific and there has been increasing utilization of normothermic bypass (48). Some surgeons will use normothermia or mild hypothermia for most of their cases, only using hypothermia during circulatory arrest. When not employing hypothermia, full-flow CPB (150 mL/kg/min) is recommended. Although the feasibility of neonatal cardiac surgery with normothermic bypass has been shown, there is still no convincing evidence of its superiority over hypothermic CPB for brain protection (49,50,51). What is known is that cerebral hyperthermia during rewarming is associated with neurocognitive dysfunction in both adults and children (52,53,54).
Deep Hypothermic Circulatory Arrest versus Continuous Low-Flow Bypass
A prospective, randomized trial performed at Boston Children’s Hospital between 1988 and 1992 assigned infants with D-transposition of the great arteries undergoing the arterial switch operation to a method of support consisting predominantly of DHCA or continuous low-flow CPB (55). This study is frequently referred to as the BCAS. Developmental and neurologic evaluation and brain MRI were performed at 1 year of age. Patients randomized to circulatory arrest had a significantly lower mean PDI score on the BSID-II. The PDI score was inversely related to the duration of DHCA, with a longer duration arrest also associated with an increased risk of neurologic abnormalities. The authors were unable to determine a safe threshold for duration of DHCA but noted that a period shorter than 41 minutes had minimal effect on the PDI score and that significant deficits were more prevalent in those infants that had a circulatory arrest period greater than 45 minutes. At 4- and 8-year follow-up, the BCAS found worse motor and speech function in the circulatory arrest group (56




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree