uncommon. It may also be seen in the absence of heart disease in the following settings:
At rest, in 25% to 35% of asymptomatic individuals under 25 years of age
In well-conditioned athletes
In some elderly patients.
Exaggerated vagal activity: Vasovagal responses may be associated with a profound bradycardia owing to heightened parasympathetic activity and sympathetic withdrawal on the sinus node. The combination of the slow heart rate and an associated decline in peripheral vascular resistance is often sufficient to produce presyncope or syncope. There are a variety of stimuli for vagal activation: Pressure on the carotid sinus, as may occur with a tight collar or with the impact of the stream of water in a shower, vomiting, or coughing, Valsalva maneuver when straining at stool, sudden exposure of the face to cold water, and prolonged standing through a Bezold-Jarisch reflex. Hypervagotonia can also result in chronic (i.e., nonepisodic) resting sinus bradycardia. This is the primary mechanism of resting bradycardia in well-trained athletes. Junctional bradycardia and Mobitz type I AV block can also be seen in this setting.
Increased intracranial pressure: Increased intracranial pressure should be excluded when sinus bradycardia occurs in a patient with neurologic dysfunction.
Acute myocardial infarction (AMI): Sinus bradycardia occurs in 15% to 25% of patients with AMI, particularly those affecting the inferior wall as the right coronary artery supplies the sinus node in approximately 60% of people. Increased vagal activity is primarily responsible, and the bradycardia is typically transient.
Obstructive sleep apnea: Individuals with obstructive sleep apnea frequently have sinus bradycardia and sinus pauses during apneic episodes. Therapies to improve the apnea frequently alleviate the bradycardia.
Drugs: A number of drugs can depress the sinus node and slow the heart rate. These include parasympathomimetic agents, sympatholytic drugs (β-blockers, reserpine, guanethidine, methyldopa, and clonidine), cimetidine, digitalis, calcium channel blockers, amiodarone and other antiarrhythmic drugs, and lithium.
Others: Other causes of sinus bradycardia include hypothyroidism, hypothermia, and severe prolonged hypoxia. Infectious agents associated with relative sinus bradycardia include Chagas’ disease, legionella, psittacosis, Q fever, typhoid fever, typhus, babesiosis, malaria, leptospirosis, yellow fever, dengue fever, viral hemorrhagic fevers, trichinosis, and Rocky Mountain Spotted fever.
TABLE 15.1 Major cause of bradycardia | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
First degree SA nodal exit block reflects a slowing of impulse exit but there is still 1:1 conduction. This abnormality cannot be recognized on the surface ECG.
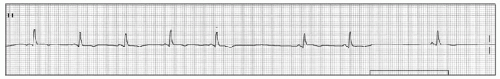
Second degree SA nodal exit block has two types. Type I (Wenckebach type) is characterized by progressively decreasing P-P intervals prior to a pause caused by a dropped P wave; the pause has a duration that is less than two P-P cycles. The mechanisms of progressive shortening of P-P interval is Wenckebach phenomenon between sinus node to atrium (Figure 15.1). In type II exit block, the P-P output is an arithmetic multiple of the presumed sinus pacemaker input (e.g., 2:1, 3:1, 4:1). Therefore the P-P cycle length surrounding the pause is a multiple of the normal P-P interval (Figure 15.2).
Third degree SA nodal exit block prevents pacemaker impulses from reaching the right atrium, giving the appearance of sinus arrest (i.e., no P waves).
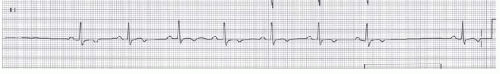 Figure 15.2. Type II second degree SA block. The rhythm strip shows a fixed P-P interval and dropped P waves similar to the rhythm strip of the upper panel. |
Increased vagal tone: Enhanced vagal tone owing to sleep, athletic training, pain, carotid sinus massage, or hypersensitive carotid sinus syndrome can result in slowing of the sinus rate and/or the development of AV block.
Idiopathic progressive cardiac conduction disease: Fibrosis and sclerosis of the conduction system accounts for about one-half of cases of AV block and may be induced by several different conditions that often cannot be distinguished clinically. Progressive cardiac conduction defect, also called Lenegre’s
or Lev’s disease, is characterized by progressive impairment of the conduction system: The term Lenegre’s disease has been traditionally used to describe a progressive, fibrotic, sclerodegenerative affliction of the conduction system in younger individuals. It is frequently associated with slow progression to complete heart block and may be hereditary. Lev’s disease has referred to “sclerosis of the left side of the cardiac skeleton” in older patients, such as that associated with calcific involvement of the aortic and mitral rings. It is caused by fibrosis or calcification extending from any of the fibrous structures adjacent to the conduction system into the conduction system. Fibrosis of the top of the muscular septum is a common cause of right bundle branch block (RBBB) with left anterior fascicular block in the elderly patient. Involvement of the mitral ring or the central fibrous body, for example, may be the commonest cause of complete heart block with a narrow QRS complex in the elderly. Aortic valve calcification, on the other hand, can invade the bundle of His, the right and/or left bundle branch as well as the left anterior fascicle. Thus, the QRS complex may be prolonged.
TABLE 15.2 Major Causes of Atrioventricular Block
Physiologic and Pathophysiologic
Iatrogenic
Increased vagal tone
Drugs
Fibrosis and sclerosis of the conduction system
digitalis, calcium channel blockers,
Ischemic heart disease
β-blockers, amiodarone
Cardiomyopathy and myocarditis
Cardiac surgery
Congenital heart disease
Transcatheter closure of VSD
Familial AV block
Alcohol septal ablation for HCM
Other
Hyperkalemia, infiltrative malignancies, neonatal lupus syndrome, severe hypo- or hyperthyroidism, trauma, degenerative neuromuscular diseases
Ischemic heart disease: Ischemic disease accounts for about 40% of cases of AV block. Conduction can be disturbed with either chronic ischemic disease or during an AMI. It is estimated that approximately 20% of patients with AMI develop AV block: 8% with first degree 5% with second degree, and 6% with third degree. Intraventricular conduction disturbances (IVCDs), including bundle and fascicular blocks, also occur in 10% to 20% of cases of acute MI. Left bundle branch block (LBBB) and RBBB with left anterior fascicle block are most common, each occurring in about one-third of patients with an IVCD. RBBB with or without left posterior fascicular block and alternating bundle branch block are less frequently seen, whereas isolated left anterior or posterior fascicle block is distinctly unusual.
Cardiomyopathy and myocarditis: AV block can be seen in patients with cardiomyopathies, including hypertrophic obstructive cardiomyopathy and infiltrative processes such as amyloidosis and sarcoidosis, and in patients with myocarditis owing to a variety of causes including rheumatic fever, Lyme disease, diphtheria, viruses, systemic lupus erythematosus, toxoplasmosis, bacterial endocarditis, and syphilis. The development of AV block in myocarditis is often a poor prognostic sign.
Congenital heart disease: Congenital complete heart block may be an isolated lesion or may be associated with other types of congenital heart disease.
Familial disease: Familial AV conduction block, characterized by a progression in the degree of block in association with a variable apparent site of block, may be transmitted as an autosomal dominant trait. Several sodium channel gene (SCN5A) mutations have been associated with sinus node and AV nodal disease. Some of these mutations produce AV block in childhood, whereas others present in middle-age and have been called hereditary Lenegre’s disease. In some families with SCN5A mutations, AV block or other conduction abnormalities are present with or without associated dilated cardiomyopathy. Different SCN5A mutations are associated with other cardiac abnormalities including congenital long QT syndrome type 3, the Brugada syndrome, familial sick sinus syndrome, and familial dilated cardiomyopathy with conduction defects and susceptibility to atrial fibrillation.
Other: AV block can also occur in a variety of other disorders:
Hyperkalemia, usually when the plasma potassium concentration is above 6.3 mEq per L.
Infiltrative malignancies, such as Hodgkin lymphoma and other lymphomas, and multiple myeloma.
Hereditary neuromuscular degenerative disease such as myotonic dystrophy, Kearns-Sayre syndrome, and Erb’s dystrophy.
Rheumatologic disorders including dermatomyositis and Paget disease.
Hyperthyroidism, myxedema, and thyrotoxic periodic paralysis.
Neonatal lupus syndrome, which results from transplacental passage of anti-Ro/SSA or anti-La/SSB antibodies from the mother.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree