Adenosine and regadenoson cause an increase in heart rate (HR) during myocardial perfusion imaging (MPI). It has been shown that patients with diabetes mellitus have a blunted HR response due to cardiac autonomic dysfunction. It is not known whether the HR response is related to hyperglycemia and the metabolic syndrome (MS). HR changes were assessed in 2,000 patients (643 with diabetes mellitus [DM]) in the Adenoscan Versus Regadenoson Comparative Evaluation for Myocardial Perfusion Imaging (ADVANCE MPI 1 and ADVANCE MPI 2) trials in relation to MS status and blood sugar level on the day of MPI. The HR response was lower in patients with MS (32.43 ± 0.52% vs 36.15 ± 0.71%, p <0.001). An increase in the number of features of MS was associated with a stepwise decrease in the HR response (−0.92% per MS criterion, p <0.05), irrespective of the presence of DM. Increasing blood sugar levels resulted in blunting of the HR response even after controlling for DM and MS (0.60 ± 0.08% per 10 mg/dl, p <0.001). MS was independently related to the HR response on top of DM, renal function, left ventricular function, gender, age, baseline HR, blood pressure, and β-blocker use. The overall model was highly associated with the HR response (p <0.001) and able to explain 30% of its variation. In conclusion, the HR response to adenosine and regadenoson is blunted in patients with hyperglycemia and in those with MS. These results suggest that factors that precede the development of DM may be associated with cardiac autonomic neuropathy and may help explain the contribution of hyperglycemia and MS to cardiovascular risk.
Adenosine and regadenoson cause a rapid increase in heart rate (HR) when used as stress agents during myocardial perfusion imaging (MPI). It has been demonstrated that this increase in HR is secondary to direct stimulation of the sympathetic nervous system by the action of these agents on the adenosine receptor A 2A . Using data from the Adenoscan Versus Regadenoson Comparative Evaluation for Myocardial Perfusion Imaging (ADVANCE MPI 1 and ADVANCE MPI 2) trials, we previously demonstrated that patients with diabetes mellitus (DM) have a blunted HR response to A 2A agonists due to cardiac autonomic dysfunction. Studies have suggested that patients who are predisposed to develop DM, such as those with the metabolic syndrome (MS) or those with impaired glucose tolerance, may have neuropathy before the development of DM. In this study, we tested the hypothesis that the HR response to adenosine and regadenoson is related to hyperglycemia and the presence of the MS.
Methods
The ADVANCE MPI trials ( CliniclTrials.gov registry numbers NCT00208299 and NCT00208312 ) randomized a total of 2,015 patients with known or suspected coronary artery disease who underwent baseline adenosine stress MPI at 109 sites in the United States and abroad to a second study with either regadenoson or repeat adenosine in a 2:1 ratio. The design and the results of the 2 trials have been previously published. Of the 2,015 patients, 15 had uncertain DM status and were excluded from this analysis. Patients had their serum blood sugar measured in the fasting state on the morning of their stress MPI studies.
Adenosine was administered intravenously as an infusion at a rate of 140 μg/kg/min for 6 minutes, and regadenoson was given as a fixed intravenous bolus dose of 400 μg. HR and blood pressure (BP) were measured at baseline and then at 2, 4, 6, 8, 10, 12, 14, 16, 30, and 45 minutes after starting the adenosine or placebo infusion.
The HR response and the BP response were calculated for each patient as the maximum percentage change from baseline, as [(HR maximum − HR baseline )/HR baseline ] × 100 and [(BP minimum − BP baseline )/BP baseline ] × 100, as previously described.
The estimated glomerular filtration rate was calculated according to the Modification of Diet in Renal Disease (MDRD) study formula : glomerular filtration rate = 32,788 × serum creatinine (μmol/L) −1.154 × age −0.203 × (0.742 if female) × (1.21 if African American).
The MS was defined as the presence of ≥3 of the 5 criteria advocated by the American Heart Association. However, because waist circumference measurements were not obtained, we substituted body mass index ≥30 kg/m 2 for the first criterion. The number of criteria satisfied was also used in the analysis. Because very few patients satisfied none or a single criterion, these patients were excluded from the linear models that were fitted.
The results of MPI were interpreted as to the presence of fixed or reversible defects, as previously described. The left ventricular ejection fraction was measured from gated single photon-emission computed tomographic perfusion images.
All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina). General linear models were used to assess the association of HR response with the described variables. Variables were considered significant if the p value based on type III sum of squares was <0.05. The following tests were used when comparing patients with versus without the MS for demographic variables, medical history, and medications. When means are compared, the differences were tested using Welch-Satterthwaite approximate t tests with no common variance assumed. The chi-square test was used when tests for association were performed. The Cochran-Mantel-Haenszel statistic was used to examine associations between ordered categories, such as glucose levels and the number of MS features satisfied, and HR response.
Results
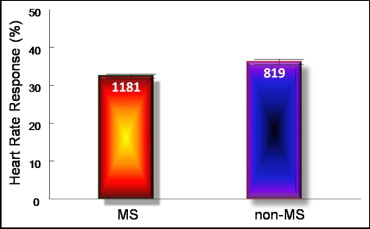
Of the 2,000 patients who constituted the study population, 1,181 (59.1%) satisfied ≥3 criteria of the MS and were considered to have the MS for the purposes of this study. The baseline characteristics and the findings on MPI of patients with and without the MS are listed in Table 1 . Patients with the MS were slightly younger, had higher body mass indexes, and were also more likely to have DM and hypertension. On MPI, the 2 populations had similar left ventricular ejection fractions, but those with the MS had a slightly higher proportion of ischemic defects.
| Variable | MS | No MS | p Value |
|---|---|---|---|
| (n = 1,181) | (n = 819) | ||
| Age (years) | 64.76 ± 0.32 | 66.51 ± 0.40 | <0.001 |
| 18–44 | 40 (3%) | 31 (4%) | |
| 45–64 | 517 (44%) | 298 (36%) | |
| 64–74 | 387 (33%) | 267 (33%) | |
| ≥75 | 237 (20%) | 223 (27%) | |
| Female gender | 409 (35%) | 193 (24%) | <0.001 |
| Race | <0.001 | ||
| Caucasian | 902 (76%) | 613 (75%) | |
| Black | 98 (8%) | 35 (4%) | |
| Hispanic | 109 (9%) | 68 (8%) | |
| Other | 72 (6%) | 103 (13%) | |
| Diabetes mellitus | 511 (43%) | 132 (16%) | <0.001 |
| Body mass index (kg/m 2 ) | 31.36 ± 0.16 | 25.92 ± 0.12 | <0.001 |
| ≤30 | 462 (39%) | 774 (95%) | |
| >30 | 719 (61%) | 45 (5%) | |
| Hypertension | 1,068 (90%) | 548 (67%) | <0.001 |
| Coronary artery disease | 916 (78%) | 631 (77%) | 0.8 |
| Glucose (mg/dl) | 109.33 ± 1.90 | 68.59 ± 1.56 | <0.001 |
| Serum creatinine (μmol/L) | 102.06 ± 1.66 | 98.05 ± 1.66 | 0.09 |
| Glomerular filtration rate (ml/min) ⁎ | 73.02 ± 0.74 | 74.88 ± 0.84 | 0.1 |
| Medications | |||
| β blockers | 198 (17%) | 164 (20%) | 0.06 |
| ACE inhibitors | 188 (16%) | 125 (15%) | 0.7 |
| Calcium channel blockers | 123 (11%) | 65 (8%) | 0.06 |
| Nitrates | 68 (6%) | 58 (7%) | 0.2 |
| Ejection fraction (%) | 55.20 ± 0.40 | 54.97 ± 0.49 | 0.7 |
| <40 | 155 (14%) | 107 (14%) | |
| 40–49 | 170 (15%) | 131 (17%) | |
| 50–59 | 356 (32%) | 230 (30%) | |
| ≥60 | 438 (39%) | 307 (40%) | |
| Perfusion | 0.006 | ||
| Fixed defect | 151 (14%) | 127 (17%) | |
| At least partially reversible defect | 569 (51%) | 348 (46%) | |
| Normal | 386 (35%) | 280 (37%) | |
| Perfusion scores | |||
| Summed rest score | 4.56 ± 0.22 | 4.96 ± 0.28 | 0.3 |
| Summed stress score | 10.35 ± 0.22 | 9.87 ± 0.26 | 0.2 |
| Summed difference score | 3.32 ± 0.14 | 2.91 ± 0.16 | 0.05 |
⁎ Calculated from serum creatinine and age on the basis of the Modification of Diet in Renal Disease (MDRD) study formula: glomerular filtration rate = 32,788 × serum creatinine (μmol/L) −1.154 × age −0.203 × (0.742 if female) × (1.21 if African American).
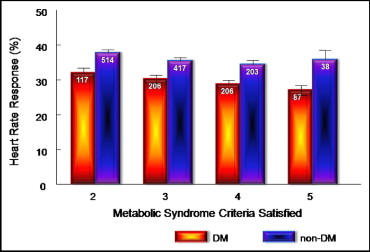
The HR response was lower in patients with the MS than in those without the MS (32.43 ± 0.52% vs 36.15 ± 0.71%, p <0.001; Figure 1 , Table 2 ). The systolic and diastolic BP responses were not different between patients with and without the MS ( Table 2 ). There was a stepwise decrease in the HR response for increasing features of the MS (34.43 ± 1.58%, 36.61 ± 0.80%, 33.63 ± 0.74%, 31.45 ± 0.83%, and 29.60 ± 1.35% for 1, 2, 3, 4, and 5 features of the MS, respectively, Cochran-Mantel-Haenszel p <0.001 accounting for −0.92% for every additional criterion met). This stepwise association was evident in patients with and those without DM ( Figure 2 ). Furthermore, an increase in the number of features of the MS in patients with DM was associated with a linear decrease in the HR response (−1.62% for every additional criterion met, p <0.05). Patients with DM had a lower HR response than patients without DM across the number of criteria of the MS satisfied (−5.92% in HR response for patients with vs without DM, p <0.001).
| Variable | Adenosine | Regadenoson | Pooled | ||||
|---|---|---|---|---|---|---|---|
| MS | No MS | MS | No MS | MS | No MS | p Value | |
| Heart rate (beats/min) | n = 387 | n = 267 | n = 770 | n = 532 | n = 1,157 | n = 799 | |
| Baseline | 67.37 ± 0.57 | 64.88 ± 0.75 | 67.08 ± 0.42 | 64.88 ± 0.49 | 67.18 ± 0.34 | 64.88 ± 0.41 | <0.001 |
| Peak | 86.00 ± 0.70 | 83.40 ± 0.94 | 89.27 ± 0.54 | 89.37 ± 0.65 | 88.17 ± 0.43 | 87.38 ± 0.54 | 0.25 |
| Heart rate response (%) ⁎ | 28.74 ± 0.82 | 29.95 ± 1.19 | 34.28 ± 0.65 | 39.27 ± 0.86 | 32.43 ± 0.52 | 36.15 ± 0.71 | <0.001 |
| Systolic BP (mm Hg) | n = 389 | n = 267 | n = 771 | n = 534 | n = 1,160 | n = 801 | |
| Baseline | 137.43 ± 0.98 | 133.41 ± 1.30 | 139.28 ± 0.74 | 134.34 ± 0.91 | 138.66 ± 0.59 | 134.03 ± 0.75 | <0.001 |
| Nadir | 122.13 ± 0.97 | 117.87 ± 1.13 | 125.44 ± 0.69 | 122.13 ± 0.79 | 124.33 ± 0.56 | 119.37 ± 0.65 | <0.001 |
| Systolic BP response (%) † | −10.91 ± 0.44 | −11.15 ± 0.57 | −9.56 ± 0.32 | −10.07 ± 0.39 | −10.01 ± 0.26 | −10.43 ± 0.32 | 0.31 |
| Diastolic BP (mm Hg) | n = 389 | n = 267 | n = 770 | n = 534 | n = 1,159 | n = 801 | |
| Baseline | 79.21 ± 0.53 | 77.17 ± 0.64 | 79.55 ± 0.39 | 76.71 ± 0.45 | 79.44 ± 0.32 | 76.86 ± 0.37 | <0.001 |
| Nadir | 68.54 ± 0.59 | 66.87 ± 0.67 | 69.68 ± 0.42 | 67.21 ± 0.43 | 69.30 ± 0.34 | 67.10 ± 0.36 | <0.001 |
| Diastolic BP response (%) † | −13.38 ± 0.52 | −13.14 ± 0.61 | −12.24 ± 0.35 | −12.07 ± 0.38 | −12.63 ± 0.29 | −12.43 ± 0.33 | 0.65 |
⁎ [(HR maximum − HR baseline )/HR baseline ] × 100.
On probing for the specific features of the MS, satisfying the criterion for elevated triglycerides or low high-density lipoprotein cholesterol did not affect the HR response, whereas satisfying the criterion for hyperglycemia, obesity, or elevated BP was associated with a lower HR response (absolute difference between patients satisfying and those not satisfying the criteria were 5.52%, 2.54%, and 3.52%, with p <0.001, p <0.003, and p <0.01, respectively). In a general linear model that included DM, both the MS and, separately, the number of criteria of the MS satisfied were significantly associated with a decrease in the HR response (p <0.05 for both). Using multiple regression, the MS was independently related to the HR response on top of DM status, glomerular filtration rate, gender, age, baseline HR, systolic BP, β-blocker use, and the left ventricular ejection fraction ( Table 3 ). The overall model was highly associated with the HR response (p <0.001) and was able to explain 30% of its variation. After adjusting for DM status, renal function, gender, age, baseline HR and BP, β-blocker use, and left ventricular systolic function, the MS independently accounted for a 3% decrease in the HR response.



