Chapter 22 Bites and Stings
Snakebites
Epidemiology
An estimated 50,000 to 100,000 individuals worldwide die each year of venomous snakebites. Those at greatest risk include agricultural workers and hunters living in tropical countries.1 In the United States, approximately 8000 bites by venomous snakes occur each year,2 with approximately six deaths. Venomous species indigenous to the United States can be found in all states except Alaska, Maine, and Hawaii. The typical victim is a young male with a bite on an extremity. Lower extremity bites tend to result from stepping near a snake, whereas purposeful handling of a snake is more likely to produce a bite on the upper extremity. Those who are purposefully handling the snake are often intoxicated. Snakes are poikilothermic, which accounts for the higher incidence of bites during warmer months.
Species
In the United States, snakes of the subfamily Crotalinae (pit vipers), which includes the rattlesnakes (Fig. 22-1), copperheads, and cottonmouths, are responsible for 99% of medically significant bites. Only 1% of bites are attributable to the other family of venomous snakes indigenous to the United States, the Elapidae (coral snakes).

FIGURE 22-1 A typical North American pit viper, the western diamondback rattlesnake, Crotalus atrox.
(Courtesy Michael Cardwell.)
Several characteristics distinguish pit vipers from nonvenomous snakes. Pit vipers tend to have relatively triangular heads, elliptical pupils, heat-sensing facial pits, large retractable anterior fangs, and a single row of subcaudal scales. Nonvenomous snakes often have more rounded heads, circular pupils, no fangs, and a double row of subcaudal scales (Fig. 22-2). Coral snakes possess a red, black, and yellow-banded pattern. In the United States, the alignment of red bands next to yellow reliably differentiates coral snakes from nonvenomous mimics. The old folk rhyme, “Red on yellow, kill a fellow, red on black, venom lack,” may overstate the problem, but is a convenient way to remember the phenotypic appearance of coral snakes in North America. There are three species of coral snakes in the United States—the eastern and Texas coral snakes (Micrurus fulvius and Micrurus tener, respectively) and the Sonoran or Arizona coral snake (Micruroides euryxanthus).
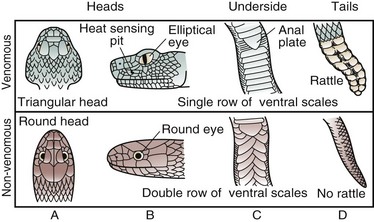
FIGURE 22-2 Comparison of pit vipers and nonvenomous snakes. The rattle (D, top panel) applies to rattlesnakes only.
(From Sullivan JB, Wingert WA, Norris RL: North American venomous reptile bites. In Auerbach PS [ed]: Wilderness medicine: Management of wilderness and environmental emergencies, ed 3, St Louis, Mosby–Year Book, 1995, p 684.)
Toxicology
Snake venoms are complex and possess many peptides and enzymes. Peptides can damage vascular endothelium, thereby increasing permeability and leading to edema and hypovolemic shock. Enzymes include proteases and L-amino acid oxidase, which cause tissue necrosis, hyaluronidase, which facilitates the spread of venom through tissues, and phospholipase A2, which damages erythrocytes and muscle cells. Other enzymes include endonucleases, alkaline phosphatase, acid phosphatase, and cholinesterase.3 In addition to causing local injury, these components also have deleterious effects on the cardiovascular, pulmonary, renal, and neurologic systems. Other components of the venom profoundly affect coagulation, fibrinolysis, platelet function, and vascular integrity, sometimes producing hemorrhagic or thrombotic sequelae.4
Clinical Manifestations
Local
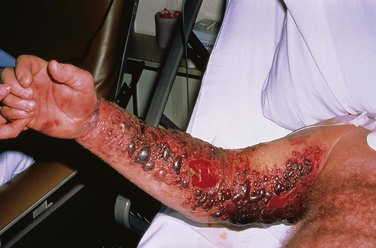
Approximately 20% of bites by pit vipers lack any venom injection (dry bites).5 The only findings in such cases are puncture wounds or lacerations and minimal pain. Actual envenomation produces burning pain within minutes, followed by edema and erythema. Swelling progresses over the next few hours, and ecchymoses and hemorrhagic bullae may appear (Fig. 22-3). Involvement of the lymphatic system is common and heralded by lymphangitis and lymphadenopathy. With delayed or inadequate treatment, severe tissue necrosis can occur.
Systemic
Patients may complain of weakness, nausea, vomiting, perioral paresthesias, a metallic taste, and muscle twitching, although these systemic complaints are uncommon.6 Diffuse capillary leakage leads to pulmonary edema, hypotension, and eventually shock. In victims of severe bites, a consumptive coagulopathy can develop within 1 hour.4 Such patients can bleed spontaneously from almost any anatomic site, although clinically significant bleeding is uncommon, even in the face of significantly abnormal coagulation test results. Multifactorial acute renal failure resulting from direct nephrotoxins, circulatory collapse, myoglobinuria, and consumptive coagulopathy is possible. Laboratory abnormalities may include hypofibrinogenemia, thrombocytopenia, prolonged prothrombin and partial thromboplastin times, increased fibrin split products, elevated creatinine and creatine phosphokinase levels, proteinuria, hematuria, and anemia or hemoconcentration.4,6
Management
Field Treatment
“Do no harm” first aid, consisting of rapid evacuation and proceeding to definitive care and immobilization, is the mainstay of first aid therapy. Removing the victim from the snake and placing him or her at rest is the first step. The wound is cleansed and immobilized at approximately heart level, if possible. Cryotherapy, suction, tourniquets, and electric shock therapy are harmful and ineffective. Therefore, these measures are not recommended. Most pit viper bites in the United States pose more of a threat to local tissues than to the life of the victim, and the use of any method to restrict venom to the bite site may be ill advised. The Australian pressure immobilization technique, in which the entire bitten extremity is snugly wrapped with a bandage, beginning at the bite site, and splinted, has been demonstrated in small studies to significantly limit the systemic spread of various snake venoms. This technique is the field treatment of choice for a non-necrotizing bite, such as from a coral snake,7 but may make the local necrosis worse after a pit viper bite. Field measures must not delay transport to the nearest hospital appropriately equipped to handle a venomous snakebite. At least experimentally, antivenom (antivenin) reduces soft tissue loss and improves function,8 and is probably time-dependent. In serious envenomations, delay in antivenin administration leads to greater tissue injury.
Hospital Management
Antivenom Therapy
Wyeth Polyvalent (Crotalidae) Antivenom is no longer manufactured. In 2000, the U.S. Food and Drug Administration (FDA) approved a second pit viper antivenom for use in the United States, CroFab (Protherics, Brentwood, Tenn). This product, produced in sheep and modified by Fab technology, appears to be more effective and safer to use than the previous polyvalent antivenin.9–11 No skin testing is recommended, and no pretreatment in an effort to reduce the risk for an acute adverse reaction to the product is needed with CroFab.
Because antivenin works by neutralizing the antigens in the venom, its dose is based on the amount of venom injected, not the size of the patient. In the clinical setting, the exact amount of venom injected is impossible to determine; therefore, the dosing of antivenin requires significant experience and clinical judgment. For major envenomations, CroFab is given as six vials intravenously (IV) in 250 mL of diluent over a period of approximately 1 hour. If, after the initial dose, the severity of venom toxicity progresses over the next hour, an additional 4 to 6 vials are administered. This sequence is repeated as needed until the victim has stabilized. After stabilization, to prevent recurrence of the effects of the venom, two vials of CroFab are administered IV every 6 hours for three additional doses.10 The same dosing regimen is used for children, and pregnancy is not a contraindication to antivenom therapy. Although expensive, postmarketing experience with CroFab has been favorable. Allergic reactions and side effects have been much less than with the previous Wyeth Polyvalent Antivenin.12
Wound Care and Blood Products
The bite site is cleansed thoroughly and the extremity is splinted and elevated. Prudent, conservative wound care is indicated. Surgical débridement is rarely indicated and should never be performed early, because injured tissue may be salvaged with antivenom administration.8 Tetanus toxoid and tetanus immune globulin are administered as needed according to the patient’s immunization history. Prophylactic antibiotics are reserved for cases in which misdirected first aid included incisions into the bite site or mouth suction. Otherwise, antibiotics are needed solely for the rare wound in which secondary infection develops.13,14
Blood products are needed only in the rare setting of clinically significant bleeding that is not reversed with antivenom. Patients with serious bleeding (e.g., gastrointestinal bleeding, intracranial bleeding, hemoptysis) may need packed red cells, platelets, fresh-frozen plasma, or cryoprecipitate, depending on the scenario and the results of serial complete blood counts and coagulation studies. Antivenom must be started before these second-line agents are infused, however.13 Patients in whom coagulopathy has developed while in the hospital after a pit viper bite must be warned that coagulation abnormalities can recur for up to 2 weeks after the bite, even after antivenom therapy. They need to be advised to look for signs of bleeding and to avoid any elective surgery or activities, with an inherent high risk of injury during this period.
Fasciotomy
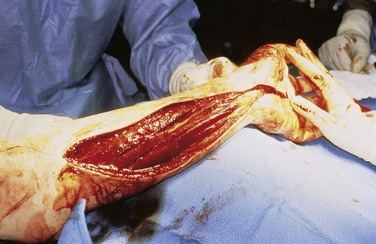
The great majority of snakebites result in the subcutaneous deposition of venom. Venom that is deposited by larger snakes into muscle compartments, however, can result in an increase in intracompartmental pressure. Subfascial deposition of venom is more likely in areas with less subcutaneous tissue (e.g., fingers, toes, anterior lower leg). Clinically differentiating a true compartment syndrome from the typical swollen, painful extremity seen after subcutaneous envenomation is difficult and may require measurement of compartment pressure. Fasciotomies are considered only if pressures are documented to exceed 30 to 40 mm Hg despite antivenom treatment and elevation (Fig. 22-4). There is no role for routine or prophylactic fasciotomy in venomous snakebites.15 Preliminary animal evidence has suggested that fasciotomy may actually increase the severity of local myonecrosis in snake venom–induced compartment syndrome.16 When a fasciotomy is required for treatment of compartment syndrome, antivenom should be aggressively administered and no débridement of injured tissue should be performed, because this tissue may be viable with antivenin therapy.8 Negative-pressure wound therapy would seem a logical choice in the postoperative care of the patient following fasciotomy for intramuscular envenomation.
Mammalian Bites
Epidemiology
The incidence of mammalian bite injuries is unknown because most patients with minor wounds never seek medical care. Although death from animal bites is uncommon in the United States, thousands of people are killed around the world each year, primarily by large animals such as lions and tigers. Dogs are responsible for 80% to 90% of animal bites in the United States, followed by cats and humans; an estimated 4.7 million dog bites occur annually in the United States and account for 1% of emergency department visits.17 Most of these bites are from a family pet or a neighborhood dog. Pit bulls and Rottweilers account for most fatal dog bites in the United States.18Animal bites occur most frequently on the extremities of adults and on the head, face, and neck of children. More than 60% of reported bites occur in children, especially boys 5 to 9 years of age.
Treatment
Wound Care
Local wound management reduces the risk for infection and maximizes functional and aesthetic outcomes. Early wound cleansing is the most important therapy for preventing infection and zoonotic diseases such as rabies. Intact skin surrounding dirty wounds is scrubbed with a sponge and 1% povidone-iodine or 4% chlorhexidine gluconate solution. Alternatively, a 1% povidone-iodine solution can be used for irrigation, as long as the wound is flushed afterward with normal saline or water. Scrubbing the wound surface itself can increase tissue damage and infection and thus needs to be avoided. Wounds that are dirty or contain devitalized tissue are cleansed lightly with gauze or a porous sponge and sharply débrided.17
Options for wound repair include primary, delayed primary, and secondary closure. The anatomic location of the bite, source of the bite, and type of injury determine the most appropriate method. Primary closure is appropriate for head and neck wounds that are initially seen within 24 hours of the bite and for which aesthetic results are important and infection rates are low.17,19,20 Primary closure can also be used for low-risk wounds to the arms, legs, and trunk if seen within 6 to 12 hours of the bite. Severe human bites and avulsion injuries of the face that require flaps have been successfully repaired by primary closure; however, this technique remains controversial. Wounds prone to the development of infection (Box 22-1), such as those initially seen longer than 24 hours after the bite (or longer than 6 hours if ear or nose cartilage is involved), are covered with moist dressings and undergo delayed primary closure after 3 to 5 days. Puncture wounds have an increased incidence of infection and are not sutured. Deep irrigation of small puncture wounds and wide excision have not proved beneficial. Larger puncture wounds, however, usually benefit from irrigation and débridement.21 Healing by secondary intention generally produces unacceptable scars in cosmetic areas. The clinician should be alert to the fact that significant dog bites may have extensive undermined areas created by the large canine teeth. These wounds require operative intervention under general or regional anesthesia.
Box 22-1 Adapted from Keogh S, Callaham ML: Bites and injuries inflicted by domestic animals. In Auerbach PS (ed): Wilderness medicine: Management of wilderness and environmental emergencies, ed 4, St Louis, 2001, CV Mosby, pp 961–978.
Animal Bite Risk Factors for Infection
Bites involving the hands or feet have a much greater chance of becoming infected and are left open.17 The primary goal in repairing bite wounds on the hand is to maximize functional outcome. Approximately one third of dog bites on the hand become infected, even with adequate therapy.21 Healing by secondary intention is recommended for most hand lacerations. After thorough exploration, irrigation, and débridement, the hand is immobilized, wrapped in a bulky dressing, and elevated.
A common human bite wound associated with high morbidity is a clenched fist injury (fight bite) resulting from striking another person’s mouth. Regardless of the history obtained, injuries over the dorsum of the metacarpophalangeal joints are treated as clenched fist injuries. These minor appearing wounds often result in serious injury to the extensor tendon or joint capsule and have significant oral bacterial contamination. The extensor tendon retracts when the hand is opened, so evaluation needs to be carried out with the hand in the open and clenched positions. Minor injuries are irrigated, débrided, and left open. Potentially deeper injuries and infected bites require exploration and débridement in the operating room and administration of IV antibiotics.22
All bite injuries are reevaluated in 1 or 2 days to rule out secondary infection.
Microbiology
Given the large variety and concentration of bacteria in mouths, it is not surprising that wound infection is the main complication of bites, with 3% to 18% of dog bite wounds approximately 50% of cat bite wounds becoming infected. Infected wounds contain aerobic and anaerobic bacteria and yield an average of five isolates/culture (Box 22-2). Although many wounds are infected by Staphylococcus and Streptococcus spp. and anaerobes, Pasteurella spp. are the most common bacterial pathogen, found in 50% of dog bites and 75% of cat bites. Human bite wounds are frequently contaminated with Eikenella corrodens in addition to the microorganisms found after dog and cat bites.22,23
Systemic diseases such as rabies, cat scratch disease, cowpox, tularemia, leptospirosis, and brucellosis can be acquired through animal bites. Human bites can transmit hepatitis B and C, tuberculosis, syphilis, and human immunodeficiency virus (HIV).20 Although HIV transmission from human bites is rare, seroconversion is possible when a person with an open wound, either from a bite or a preexisting injury, is exposed to saliva containing HIV-positive blood.24 In this scenario, baseline and 6-month postexposure HIV testing is performed and prophylactic treatment with anti-HIV drugs is considered.
Antibiotics
Although data are limited, preventive antibiotics are recommended for patients with high-risk bites.17,21 The initial antibiotic choice and route are based on the type of animal and severity and location of the bite. Cat bites often cause puncture wounds that require antibiotics. Patients with low-risk dog and human bites do not benefit from prophylactic antibiotics unless the hand or foot is involved.23 Patients seen 24 hours after a bite without signs of infection do not usually need prophylactic antibiotics. Routine cultures of uninfected wounds have not proved useful and are reserved for infected wounds.
Initial antibiotic selection needs to cover Staphylococcus and Streptococcus spp., anaerobes, Pasteurella spp. for dog and cat bites, and E. corrodens for human bites. Amoxicillin-clavulanate is an acceptable first-line antibiotic for most bites. Alternatives include second-generation cephalosporins, such as cefoxitin, or a combination of penicillin and a first-generation cephalosporin. Penicillin-allergic patients can receive clindamycin combined with ciprofloxacin (or combined with trimethoprim-sulfamethoxazole if the patient is pregnant or a child).17,20 Moxifloxacin has also been suggested as monotherapy. Infections developing within 24 hours of the bite are generally caused by Pasteurella spp. and are treated by antibiotics with appropriate coverage. Patients with serious infections require hospital admission and parenteral antibiotics such as ampicillin-sulbactam, piperacillin-tazobactam, cefoxitin, ticarcillin-clavulanate or clindamycin combined with a fluoroquinolone, or trimethoprim-sulfamethoxazole.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree