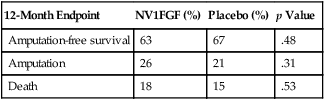
FGF has been extensively studied in the context of CLI. The TALISMAN phase II trial (Clinicaltrials.gov NCT00798005) enrolled 125 patients and reported a significant improvement in amputation-free survival of 52% in placebo-treated patients with no options for revascularization compared to 73% in patients treated with FGF plasmid (p = .009). In a separate study, these investigators showed proof of concept of gene therapy when they identified the FGF plasmid, mRNA, and protein in the affected extremities of patients with CLI who received FGF plasmid injections before amputation. These findings led to completing a phase III pivotal trial, the TAMARIS trial (NCT00566657). Unlike the earlier phase II trial, the TAMARIS trial failed to show a difference in amputation-free survival when compared to placebo in patients with CLI (Table 1). This trial enrolled 525 patients at 170 sites in more than 30 countries. Subjects had either a hemodynamically confirmed ischemic ulcer or minor gangrene. Major amputation or death at 1 year occurred in 33% of placebo-treated patients and 36% of treated patients. The amputation-free survival for both groups was similar to that for the FGF-treated patients in the phase II TALISMAN trial. The likely explanation for the different results observed in the phase II TALISMAN and phase III TAMARIS trials is a type II error. TABLE 1 TAMARIS Trial: NV1FGF Plasmid versus Placebo (525 Patients) There are currently no FDA-approved gene therapies to treat patients with CLI. Cellular therapies can be divided into autologous and allogeneic. Several phase I and II trials have been completed, including Harvest Technologies (NCT00498069) and Biomet, both of which have reported promising early results of phase II trials using autologous bone marrow mononuclear cells (BMNC) in the treatment of CLI. Both companies have developed point-of-care cell-preparation systems that, following bone marrow harvest of 240 to 300 mL, allows separation of the cells and extraction of the BMNC component for direct intramuscular injection into the ischemic limb. Based on promising early results, both companies have begun phase III trials and are performing these trials through Investigator Device Exemptions (IDE) from the Center for Device and Radiologic Health (CDRH) of the Food and Drug Administration. Based on their phase II trial, in which major amputations occurred in 18% of treated patients compared to 29% in placebo-treated patients, Harvest Technologies has initiated a phase III trial (NCT01245335) (Table 2). Murphy and coworkers have also shown amputation-free survival of 86% in a similar open-label trial using autologous bone marrow cells in patients with rest pain or ischemic ulcers. TABLE 2 Harvest Technologies Phase II Trial: Bone Marrow Aspirate Concentrate (48 Patients)
Biologic Therapies for Patients with Critical Limb Ischemia
Gene Therapy Trials
12-Month Endpoint
NV1FGF (%)
Placebo (%)
p Value
Amputation-free survival
63
67
.48
Amputation
26
21
.31
Death
18
15
.53
Stem Cell Therapy Trials
3-Month Endpoint
Bone Marrow Concentrate (n = 34) (%)
Control (n = 14) (%)
p Value
Major amputation
17.6
28.6
.45
Improved pain
44
25
.54
Improved ABI
32
7
.08
Improved Rutherford Classification
35
14
.18 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Biologic Therapies for Patients with Critical Limb Ischemia
