20 Bifurcations and Branch Vessel Stenting
 Bifurcation Lesions
Bifurcation Lesions
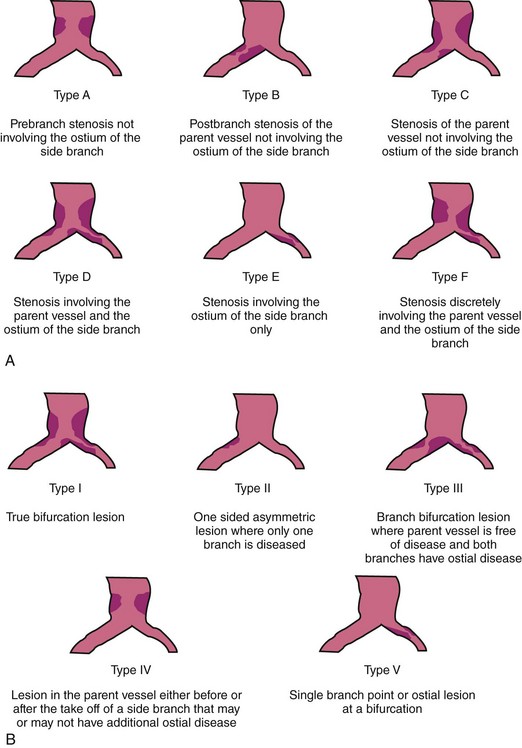
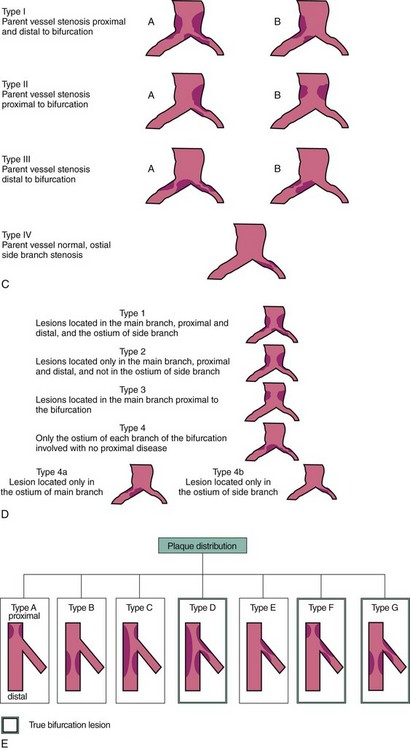
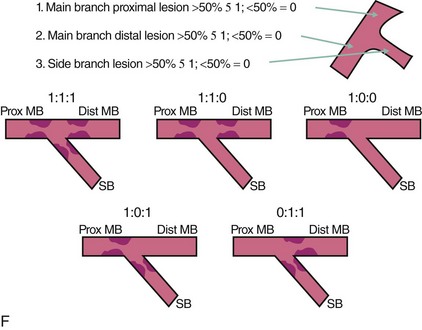
A bifurcation coronary lesion occurs at or adjacent to a significant division of a major epicardial coronary artery. Coronary bifurcation lesions have been the subject of several classifications with the underlying assumption that each type could be associated with a specific treatment. However, attempts to classify bifurcation lesions have all of the limitations of coronary angiography (different plaque distribution and extent of disease when evaluated by intravascular ultrasound).1–6 At the present time, there are six different classifications of bifurcation lesions (Fig. 20-1). The most important distinction is to divide bifurcation lesions into “true” bifurcations, where the main branch (MB) and the side branch (SB) are both significantly narrowed (>50% diameter stenosis), and “nontrue” bifurcations, which include all the other lesions involving a bifurcation. In routine practice, the Medina classification is still the simplest and most widely used approach to classify the distribution of atherosclerotic plaque at the bifurcation site.5 The designation “1” means the presence of stenosis and “0” the absence of stenosis, in each of the three bifurcation segments, starting with proximal MB, distal MB, and proximal SB: the designation “1.1.1” indicates a critical stenosis in all three segments and “1.1.0” when only the proximal and distal MBs are affected; however, many other combinations are possible. A limitation of the Medina classification is that the length of the stenosis involving the SB is not specified; this distinction is, however, a key element to properly plan the treatment. Despite an array of devices available, the use of DESs remains the default approach to treat bifurcation lesions, and the implantation of a single stent on the MB is the most widely used approach.
Contemporary Studies
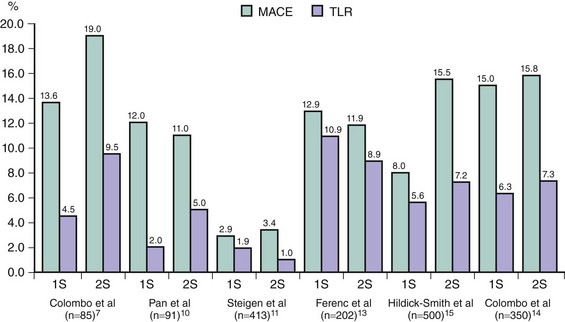
Several major randomized trials comparing the use of one or two stents in the treatment of coronary bifurcations demonstrated that the implantation of a stent only in the MB remains the preferred strategy. The sirolimus-eluting stent (SES) bifurcation study has been the first attempt to provide specific information in this subset of lesions.7 Eighty-five patients were randomly assigned to either stenting both branches or stenting the MB only with provisional stenting of the SB. Data were analyzed by actual treatment received, not by intention to treat. The cross-over rate was very high, 51.2% in the provisional group and 4.7% in the two-stent group. Re-stenosis at 6 months did not differ significantly between the two-stent (28.0%) and the stent-plus-percutaneous transluminal coronary angioplasty (PTCA) (18.7%) groups (P = 0.53). During the 6-month follow-up, there was one death in the two-stent group and none in the stent-plus-PTCA group. There was no significant difference between the groups in Q wave myocardial infarction (MI) (1.6% vs. 4.5%), non–Q wave MI (9.5% vs. 4.5%), tricuspid valve regurgitation (TVR) (11.1% vs. 9%), or target vessel failure (19% vs. 13.6%). There were three cases of stent thrombosis, all of which occurred in the stent–stent group. Therefore, this study demonstrated that, compared with historical studies using bare metal stents (BMSs), a clear improvement has been achieved in the treatment of bifurcation lesions when one or two DESs were implanted.1,8,9 In a second study, Pan and coworkers compared two strategies for the SES treatment of bifurcation lesions in 91 patients with “true” coronary bifurcation lesions.10 Major adverse cardiac events (MACE) at six months were similar between two groups and occurred in 3 patients in the provisional group (2 non–Q wave MIs and 1 TLR) and in 3 patients in the two-stent group (1 subacute stent thrombosis with subsequent death and 2 TLR). Six-month angiographic re-evaluation was obtained in 80 patients (88%) and the re-stenosis rates of the MB and the SB were also similar in two study groups. A third randomized study was the Nordic Bifurcation Study (Nordic), in which 413 patients were randomized to two stents (n = 206) using crush, culotte, Y, or other techniques or provisional stenting (n = 207) with SES implantation.11 The cross-over from provisional stenting to two stents was allowed only with thrombolysis in MI (TIMI) flow grade 0 following SB dilation. Procedural success was achieved in 97% of cases in provisional stenting versus 95% in the two-stents group. The cross-over rate was also low, and SB was stented only in 4.3% of the patients in the provisional stenting group. Final kissing balloon inflation was performed in 32% and 74% of the patients, respectively (P < 0.001). At 6 months, there was no difference between the two groups with regard to cardiac death, MI, index lesion MI, TVR, TLR, and stent thrombosis. Fourteen-month follow-up confirmed these results, and 2 months after recommended cessation of dual anti-platelet therapy, the rates of stent thrombosis and MACE were low and similar in the two arms.12 In a fourth trial, Bifurcations Bad Krozingen (BBK) study, Ferenc et al compared 202 patients randomly allocated to either provisional T stenting or routine T stenting, using SES.13 Final kissing balloon dilatation was performed in both groups irrespective of whether they were assigned to routine or provisional T stenting; in the provisional T stent arm, cross-over to SB stenting was mandated in case of a flow-limiting dissection or residual stenosis of greater than 75%. The primary endpoint was percent diameter stenosis of the SB at 9-month angiographic follow-up and was similar between the two groups (P = 0.15). The overall 1-year incidence of target lesion re-intervention was 10.9% after provisional T stenting and 8.9% after routine T stenting (P = 0.64), and the primary clinical outcome (death, MI, target lesion revascularization) was equivalent in both groups (12.9% provisional T stenting vs. 11.9% routine T stenting (P = 0.83). The fifth trial of interest was the CACTUS trial (Coronary Bifurcations: Application of the Crushing Technique Using Sirolimus-Eluting Stents).14 In this study, 350 patients were randomly allocated to either provisional T or crush SES implantation, with mandatory final kissing balloon inflation in both groups. Stent implantation in the SB was allowed by the T stenting technique only when at least one of the following conditions was met: (1) residual stenosis 50% or greater, (2) dissection of type B or worse, or (3) thrombolysis in MI (TIMI) flow grade 2 or less. A high proportion of patients (92%) had true bifurcations, and the cross-over rate in the provisional T group was 31%. The primary angiographic endpoint was the in-segment re-stenosis rate, and the primary clinical endpoint was a 6-month rate of major adverse cardiac events (cardiac death, MI, or target vessel revascularization). At 6 months, angiographic re-stenosis rates were not different between the crush group (4.6% and 13.2% in the MB and SB, respectively) and the provisional stenting group (6.7% and 14.7% in the MB and SB, respectively; P = not significant [NS]). The primary clinical outcome (death, MI, revascularization) was also similar in both groups (15% provisional vs. 15.8% crush, P = NS). Finally, in the BBC ONE study (British Bifurcation Coronary Study: Old, New, and Evolving Strategies), 500 patients were randomly allocated to either a simple strategy (minimalist provisional T) or a complex strategy (either crush or culotte) using paclitaxel-eluting stents (PES).15 In the simple strategy, the MB was stented, followed by optional kissing balloon dilatation or T stent, using the following criteria: TIMI flow grade less than 3 in the SB, severe ostial pinching of the SB (>90%), threatened SB closure, or SB dissection greater than type A. If one of these criteria existed, the operator could progress to the next stage, but if the SB was stented, final kissing balloon was mandatory. Only 30% of the SB underwent further treatment after MB stenting (27% balloon dilatation and 3% stenting). In the complex strategy, both branches were systematically stented (culotte or crush techniques) with mandatory kissing balloon dilatation. At 9 months clinical follow-up, there was a significant difference between the two groups in terms of death, MI, or revascularization (simple 8% vs. complex 15.5%). This difference was largely driven by the higher incidence of MI in the complex group (11.2% vs. 3.6%, P = 0.001). It is quite surprising to note the very high number of complications that occurred in patients randomized to the complex strategy. The clinical implication of those trials is that in most cases, provisional stenting rather than elective stenting of the SB should be performed. Provisional stenting seems to be less expensive and simpler and can be performed with less contrast and in a shorter procedural time. At the same time, the evidence of equivalent results in the two arms does not contradict the choice of crush or culotte stenting in selected cases with complex anatomy or with diffuse disease in the SB. The consensus from randomized trial data was that routine two-vessel stenting did not improve either angiographic or clinical outcomes for most patients with coronary bifurcation lesions, although routine two-vessel stenting did not involve a significant penalty either.
Clinical outcomes in randomized trials comparing one-stent versus two-stent strategy that included DESs are presented in Fig. 20-2. To evaluate the impact of a specific two-stent technique, the Nordic complex bifurcation stenting study was performed. In this investigation, 424 patients were randomized to either crush or culotte stenting using SES (77% of which were “true” coronary bifurcation lesions).16 At 6 months clinical follow-up, there was no difference between the two groups in terms of death, post-procedure MI, or revascularization (the primary endpoint: crush 4.3% vs. culotte 3.7%, P = 0.87). However, there was a trend toward higher incidence of peri-procedural MI (crush 15.5% vs. culotte 8.8%, P = 0.08) and significantly higher occurrence of in-stent re-stenosis in the crush group (crush 10.5% vs. culotte 4.5%, P = 0.046). To evaluate the impact of a specific DES type on clinical outcome in patients with bifurcation lesions, Pan et al enrolled 205 patients in a prospective randomized trial; 103 patients were assigned to SES and 102 patients to PES.17 All patients were treated with provisional T stenting. Angiographic data and immediate procedural results were similar in both groups. There was no difference in the rates of death or MI in hospital and during follow-up. The primary endpoint, the angiographic re-stenosis rate, was significantly lower in the SES group (9% vs. 29%, P = 0.011), as was target lesion revascularization at 24 months after stenting (4% vs. 13%, P = 0.021). Similar results were obtained in the COBIS (Coronary Bifurcation Stenting) registry, which compared major adverse cardiac events (MACE defined as cardiac death, MI, or target lesion revascularization) between SES and PES.18 At a mean follow-up of 22 months, treatment with SES resulted in a lower incidence of MACE (HR 0.53; 95% CI 0.32–0.89, P < 0.01) and target lesion revascularization (HR 0.55; 95% CI 0.31–0.97, P = 0.02) but not of cardiac death and cardiac death or MI.
Approach to Treatment of Bifurcation Lesions
Bifurcations vary not only in anatomy (plaque burden, location of plaque, angle between branches, diameter of branches, bifurcation site) but also in the dynamic changes in anatomy during treatment (plaque or carina shift, dissection). Previous pathologic studies demonstrated that atherosclerosis occurs predominantly in low shear–stress regions of bifurcation but that carina (flow divider) involvement by atherosclerosis is extremely unusual. Nakazawa et al recently demonstrated that in nonstented coronary bifurcations, the lateral wall showed significantly greater intima as well as necrotic core thickness compared with the flow divider.19 After stenting, plaque formation and neointimal growth were also significantly less at the flow divider compared with the lateral wall. Those observations were also confirmed in vivo with the IVUS (intravenous ultrasound) preintervention evaluation of the distal left main coronary artery (LMCA) bifurcations.20 Oviedo et al showed that bifurcation disease is usually diffuse and, contrary to angiographic classifications, that the carina (flow divider) was spared in all lesions, whereas plaque was present predominantly on the opposite side of the flow divider. However, data presented by van der Giessen et al threw new light on the subject through computed tomographic (CT) angiography, which suggested that the carina does have some atheroma present in 30% of bifurcations, though the volume of that plaque remains small and atheroma was present only if there is significant volume of plaque located at the lateral walls opposite to flow divider (low wall shear–stress areas), indicating that atherosclerotic plaque grows circumferentially.21 Koo et al evaluated with IVUS and fractional flow reserve (FFR) the mechanisms of changes in the geometry of the ostium of the SB after MB stenting to test the hypothesis that MB stenting may create worsening of an SB ostial lesion as a result of a combination of MB plaque and carina shift.22 The investigators concluded that the decrease in plaque volume in the proximal MB, with no associated increase in plaque volume in the distal MB, was indirect evidence of plaque shift from the MB to the SB ostium after stent implantation. Additionally, the increased luminal volume in the distal MB, with no significant decrease in the plaque volume, was believed to be caused by vessel enlargement and provided support to the theory that carina shift is likely to contribute to the degree of luminal narrowing of the SB. As a result of those investigations, it can be concluded that no two bifurcations are identical and that no single strategy can be applied to every bifurcation. Thus, the more important issue in bifurcation PCI is selecting the most appropriate strategy for an individual bifurcation and optimizing the performance of this technique.
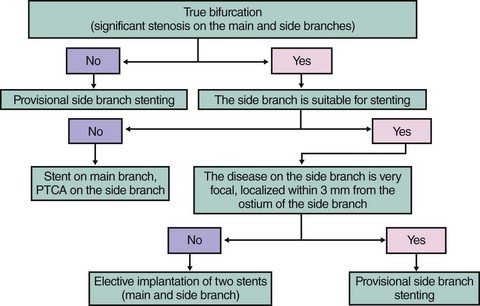
General Outline for Treating a Bifurcation Lesion
Fig. 20-3 summarizes a proposed approach to bifurcation lesions with an attempt to give directions for SB stenting as intention to treat only in cases where disease on the SB extends beyond the ostium and the SB has a significant area of distribution.
The most frequently used approach is provisional SB stenting, and it is outlined as follows:
Difficult Access to the Side Branch
After having attempted different types of wires with all sorts of curves and exhausted all personal tactics, the operator may still be unable to advance a wire in the SB. At this point, few options are available: (1) aborting the procedure because the risk of losing the SB will be too high, depending on the size and distribution of the branch (typically an angulated circumflex artery), (2) performing rotational atherectomy on the MB with the intent to remove the plaque that prevents entry toward the SB, (3) dilating the MB with a balloon on the basis of the rationale that plaque modification and, hopefully, a favorable plaque shift will facilitate access toward the SB, (4) using the Venture wire control catheter (VWC-St. Jude Medical, Maple Grove, MN), a low-profile catheter with a tip that can be deflected up to 90 degrees, which facilitates wire orientation and provides excellent backup support (Fig. 20-4).23 Each of these four options has its rationale, and the specific anatomic condition, the operator’s experience, and the clinical scenario may direct the selection of the best strategy. Usually, the third option is the one more frequently employed and is effective most of the time.
Role of Final Kissing Balloon Inflation
Final kissing balloon inflation is proposed if the SB is dilated through the MB stent struts to correct MB stent distortion and proximal expansion and to provide better scaffolding of the SB ostium and facilitate future access to the SB. The CACTUS trial subanalysis showed that final kissing balloon inflation was associated with better angiographic results and lower MACE rates when complex stenting was performed and similar results were observed when using a simpler provisional SB stenting technique.14 Several criteria have been proposed to define lesions in which final kissing balloon inflation is required (>75% residual stenosis at the SB, TIMI flow grade <3, or FFR <0.75). However, there is significant debate on the use of routine kissing inflations following the Nordic III study presentation during the TCT 2009 meeting, which has established that there is no systematic clinical advantage to a routine kissing strategy when a single stent treatment is used (http://www.tctmd.com/show.aspx?id=84126; accessed on June 12, 2010). Therefore, two appropriate strategies are (1) to use a pressure wire to interrogate the significance of the side branch lesion and to treat or not treat, accordingly, or (2) simply to do kissing balloon inflations on all angiographically significant ostial SB lesions, which reduces the proportion of physiologically significant SB ostial lesions; this is supported by information from the Nordic III trial which demonstrated that there appears to be no penalty for doing so.24 A study by Koo et al has shown that kissing balloon inflation restores normal FFR.25 As a general approach, the authors of this chapter favor final kissing balloon inflation.
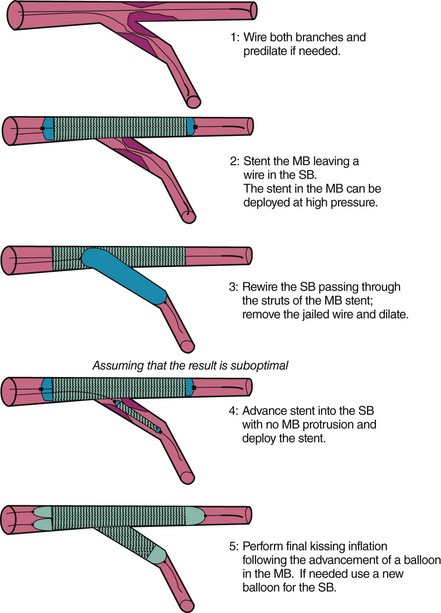
A Second Stent in the Side Branch Following the Provisional Approach
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree