Beta blockers have been used in the treatment of cardiovascular conditions for decades. Despite a long history and status as a guideline-recommended treatment option for hypertension, recent meta-analyses have brought into question whether β blockers are still an appropriate therapy given outcomes data from other antihypertensive drug classes. However, β blockers are a heterogenous class of agents with diverse pharmacologic and physiologic properties. Much of the unfavorable data revealed in the recent meta-analyses were gleaned from studies involving nonvasodilating, traditional β blockers, such as atenolol. However, findings with traditional β blockers may not be extrapolated to other members of the class, particularly those agents with vasodilatory activity. Vasodilatory β blockers (i.e., carvedilol and nebivolol) reduce blood pressure in large part through reducing systemic vascular resistance rather than by decreasing cardiac output, as is observed with traditional β blockers. Vasodilating ability may also ameliorate some of the concerns associated with traditional β blockade, such as the adverse effects on metabolic and lipid parameters, including an increased risk for new-onset diabetes. Furthermore, vasodilating ability is physiologically relevant and important in treating a condition with common co-morbidities involving metabolic and lipid abnormalities such as hypertension. In patients with hypertension and diabetes or coronary artery disease, vasodilating β blockers provide effective blood pressure control with neutral or beneficial effects on important parameters for the co-morbid disease. In conclusion, it is time for a reexamination of the clinical evidence for the use of β blockers in hypertension, recognizing that there are patients for whom β blockers, particularly those with vasodilatory actions, are an appropriate treatment option.
Building on the availability of propranolol since 1976, more than a dozen additional β blockers have been introduced for hypertension treatment. This antihypertensive drug class effectively lowers blood pressure and has been a recommended treatment option by the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. However, in the recent National Institute for Health and Clinical Excellence hypertension guidelines, β blockers were no longer given first-line status because of concerns driven by outcomes data. Concerns have also been raised by meta-analyses in which β blockers were reported to have a suboptimal effect on reducing stroke risk and increasing the risk for new-onset diabetes compared with other antihypertensive agents. However, a closer examination of trials included in Lindholm et al’s meta-analysis revealed that the primary β blocker evaluated was atenolol, a traditional β blocker, and results for all other β blockers were insufficient for any definite conclusions. Moreover, the new-onset diabetes meta-analysis evaluated primarily atenolol, metoprolol, and propranolol, all β blockers without vasodilatory activity. Vasodilatory activity may be a key contributor to advantageous outcomes in hypertension. This review examines the current evidence supporting the use of β blockers in the treatment of hypertension, focusing on class diversity, recent vasodilatory β-blocker data, and compelling indications for β blockers.
Traditional β Blockers in the Treatment of Hypertension
There is a misconception that β blockers do not lower blood pressure equivalently to other classes of antihypertensive agents. However, the Blood Pressure Lowering Treatment Trialists’ Collaboration meta-analysis reported that among 8 trials involving 37,872 patients comparing different classes of antihypertensive agents (angiotensin-converting enzyme [ACE] inhibitors, calcium antagonists, and β blockers and/or diuretics), blood pressure differences between treatment groups during follow-up (2 to 8 years) were minimal (systolic blood pressure [SBP] 0 to 3 mm Hg, diastolic blood pressure [DBP] <1 to 2 mm Hg). Notably, β blockers were combined with diuretics as a single comparator group in the meta-analysis because of frequent concomitant use; the effects of β blockers alone were not reported. However, blood pressure reductions in large clinical trials comparing β blockers with diuretics showed no statistically significant differences between the 2 treatments, with approximately 3/4 of patients receiving either drug class achieving DBP goals. It should be acknowledged that add-on therapy was more common with diuretics than with β blockers in these studies.
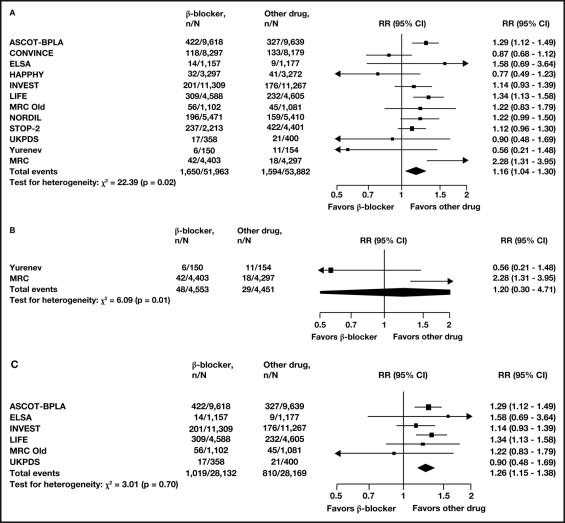
As noted in the National Institute for Health and Clinical Excellence guidelines, β blockers, primarily atenolol, were less effective than other classes of antihypertensive agents in reducing clinical complications, especially stroke. However, to extrapolate these results to other agents in the β-blocker class is unwarranted, because there is a lack of quantitative evidence (i.e., outcomes data). In a meta-analysis of 7 trials involving 27,433 patients by Lindholm et al, a reduction in stroke risk was reported with traditional β blockers, primarily atenolol, versus placebo or no treatment (about 19%). However, the risk reduction was not comparable with the reductions reported from 12 trials (9 trials with atenolol) involving 105,845 patients that compared ACE inhibitors, calcium antagonists, angiotensin receptor blockers (ARBs), and/or diuretics with β blockers (16% higher risk for β blockers, p = 0.009; Figure 1 ). In contrast, differences in risk for myocardial infarction (relative risk 1.02, 95% confidence interval 0.93 to 1.12) and overall mortality (relative risk 1.03, 95% confidence interval 0.99 to 1.08) were not statistically significant between β blockers and other antihypertensive classes. A limitation of this meta-analysis is that significant heterogeneity was present in the patient population. Another meta-analysis by Wang and Staessen (9 trials involving 62,605 patients) reported no statistically significant difference between newer classes of antihypertensive agents (calcium channel blockers [CCBs], ACE inhibitors, and ARBs) and older agents (diuretics and β blockers) for cardiovascular mortality. A lack of substantial data from trials with β blockers other than atenolol would make it difficult to state broad outcome claims for β blockers as a class.
Traditional β blockers such as atenolol, metoprolol, and propranolol reduce blood pressure primarily via the reduction of cardiac output through chronotropic and inotropic inhibitory mechanisms. However, reduced cardiac output can induce compensatory peripheral vasoconstriction to maintain blood pressure, which abets increased peripheral resistance, a hallmark of chronic hypertension. An increase in systemic vascular resistance diminishes blood flow to peripheral tissues, such as the skeletal muscles, and may lead to adverse effects on lipid and glucose metabolism, which in turn contribute to the development of endothelial dysfunction and diabetes.
Although traditional β blockers effectively lower brachial (arm) blood pressure, recent clinical data suggest that they may have less effect on reducing central aortic pressure compared with other antihypertensive classes. In the Conduit Artery Function Evaluation (CAFE), a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), central aortic pressure and brachial SBP were evaluated in 2,199 patients with hypertension and ≥3 other cardiovascular risk factors. Despite a difference of only 0.7 mm Hg in brachial SBP reduction between the amlodipine-based and atenolol-based therapy, favoring amlodipine, central aortic SBP was decreased by 4.3 mm Hg with amlodipine compared to atenolol (p <0.0001; Figure 2 ). Because increased central aortic pressure has been associated with an increased risk for vascular events, especially stroke, these results may at least partially explain the residual stroke risk associated with traditional β blockers.

Vasodilatory β Blockers in Treating Hypertension
Vasodilatory β blockers decrease blood pressure largely through reducing systemic vascular resistance, while maintaining cardiac output. The benefits of peripheral vasodilation contribute to reduced cardiac afterload and preload, lack of adverse effects on lipid and glucose metabolism, and possible reversal of adverse arterial remodeling. Arterial remodeling (stiffness) may increase distal wave reflection of blood back to the aorta, which augments the outgoing central systolic pulsewave from the heart, thus increasing central aortic pressure. Reversal of arterial remodeling may thereby lower central aortic pressure. By lowering blood pressure in a more physiologically relevant manner, vasodilatory β blockers may be a more appropriate therapy for hypertension compared with traditional β blockers ( Table 1 ).
| Effect | Ideal Drug | Traditional β Blocker | Vasodilating β Blocker | α 1 Adrenoceptor Blocker | ACE Inhibitor/ARB | DHP Calcium Antagonist | Thiazide Diuretic |
|---|---|---|---|---|---|---|---|
| Mean arterial blood pressure | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Total peripheral resistance | ↓ | (↑) | (↓) | ↓ | ↓ | ↓ | ↓ |
| Cardiac output | 0 | (↓) | 0 | 0 | 0 | 0 | 0 |
| Heart rate | 0/↓ | ↓ | 0/↓ | (↑) | 0 | (↑) | 0 |
| SNS activation | ↓ | ↓ | ↓ | (↑) | ↓ | ↑ | ↑ |
| RAS | ↓ | ↓ | ↓ | 0 | ↓ | ↑ | ↑ |
| Lipid metabolism | 0/+ | − | 0 | 0/+ | 0 | 0 | − |
| Glucose metabolism | 0/+ | − | 0 | 0 | 0/+ | 0 | − |
Labetalol
Labetalol is a nonselective β locker with α 1 receptor–blocking activity and minimal intrinsic sympathomimetic activity. Labetalol lowers blood pressure rapidly (orally, within 2 hours; intravenously, within 5 minutes) and therefore has been useful in hypertensive emergencies. A randomized, double-blind, placebo-controlled clinical trial in 74 patients with mild hypertension showed that labetalol (600 mg/day) effectively lowered supine and standing blood pressure compared to placebo (p <0.05). In 134 patients with moderate to severe hypertension (DBP 105 to 129 mm Hg), open-label labetalol (100 to 400 mg/day for up to 18 weeks) effectively lowered standing blood pressure from baseline (−32.9/−20.4, p <0.001 for both) with 75% of patients achieving goal blood pressure (DBP ≤90 mm Hg). Labetalol was generally well tolerated in these clinical studies. On the basis of data from small clinical trials, labetalol was equally effective in lowering SBP and more effective in lowering 24-hour DBP compared to a CCB or an ACE inhibitor.
Carvedilol
Carvedilol is a nonselective β blocker with α 1 receptor–blocking activity and no intrinsic sympathomimetic activity. Clinical data suggest that carvedilol reduces systemic vascular resistance in patients with hypertension. Carvedilol was originally formulated for twice-daily administration; however, a bioequivalent, once-daily, controlled-release carvedilol formulation is now available for the same clinical indications (hypertension, heart failure, and post–myocardial infarction left ventricular dysfunction). In a placebo-controlled, double-blind trial in 338 patients with essential hypertension randomized to controlled-release carvedilol 20, 40, or 80 mg or placebo once daily for 6 weeks, each controlled-release carvedilol dose significantly lowered mean 24-hour SBP and DBP assessed by ambulatory blood pressure monitoring compared with placebo (p ≤0.001 for all). Blood pressure reductions were dose dependent and maintained throughout the dosing interval ( Figure 3 ). Controlled-release carvedilol was generally well tolerated, with an overall similar low incidence of emergent adverse events as observed with placebo.
Nebivolol
Nebivolol is a β 1 -selective β blocker that does not have α 1 -blocking activity or intrinsic sympathomimetic activity. Clinical data suggest that nebivolol reduces systemic vascular resistance in patients with hypertension, possibly through stimulation of nitric oxide release. A recent randomized, double-blind, placebo-controlled trial in 909 patients with mild to moderate hypertension showed that nebivolol 1.25 to 40 mg/day effectively lowered SBP and DBP compared to placebo after 84 days of treatment (p ≤0.002 and p <0.001, respectively, for all doses; Figure 4 ). In an open-label, 6-week trial in 6,356 patients with mild hypertension (defined as DBP of 90 to 115 mm Hg) and mean baseline SBP of 162 mm Hg, nebivolol (5 to 10 mg/day) significantly lowered mean SBP and DBP from baseline (−24 and −13 mm Hg, respectively, p <0.001 for both). Nebivolol (2.5 to 10 mg/day) was also assessed as monotherapy or as add-on therapy in 2,838 patients with hypertension and type 2 diabetes mellitus for ≥3 months and lowered SBP and DBP from baseline by 21 and 11 mm Hg, respectively. Although most patients receiving nebivolol 5 mg/day achieved blood pressures ≤140/90 mm Hg, only 9.6% of patients achieved the goal blood pressure recommended for patients with diabetes (<130/80 mm Hg). Nebivolol was generally safe and well tolerated. Nebivolol had comparable efficacy with other β blockers and antihypertensive agents. In a meta-analysis of 12 randomized clinical trials in patients with hypertension, achievement of blood pressure targets or goal reductions with nebivolol was higher than that of ACE inhibitors (odds ratio 1.92, p = 0.001) and similar to that of other β blockers, CCBs, and losartan.
Vasodilatory β Blockers in Treating Hypertension
Vasodilatory β blockers decrease blood pressure largely through reducing systemic vascular resistance, while maintaining cardiac output. The benefits of peripheral vasodilation contribute to reduced cardiac afterload and preload, lack of adverse effects on lipid and glucose metabolism, and possible reversal of adverse arterial remodeling. Arterial remodeling (stiffness) may increase distal wave reflection of blood back to the aorta, which augments the outgoing central systolic pulsewave from the heart, thus increasing central aortic pressure. Reversal of arterial remodeling may thereby lower central aortic pressure. By lowering blood pressure in a more physiologically relevant manner, vasodilatory β blockers may be a more appropriate therapy for hypertension compared with traditional β blockers ( Table 1 ).



