Bivalirudin, a direct thrombin inhibitor, has been shown to reduce major bleeding and provide a better safety profile compared to unfractionated heparin (UFH) in patients undergoing percutaneous coronary intervention (PCI) through transfemoral access. Data pertaining to the clinical benefit of bivalirudin compared to UFH monotherapy in patients undergoing transradial PCI are lacking. The present study sought to compare the in-hospital net clinical adverse events, including death, myocardial infarction, target vessel revascularization, and bleeding, for these 2 antithrombotic regimens for all patients at a tertiary care, high-volume radial center. From April 2009 to February 2011, all patients treated with bivalirudin were matched by access site to those receiving UFH. The patients in the bivalirudin group (n = 125) were older (72 ± 13 years vs 66 ± 11 years; p <0.0001), more often had chronic kidney disease (51% vs 30%; p = 0.0012), and more often underwent primary PCI (30% vs 14%, p <0.0037) than the UFH-treated patients (n = 125). A radial approach was used in 71% of both groups. The baseline bleeding risk according to Mehran’s score was similar in both groups (14 ± 9 vs 15 ± 8, p = 0.48). In-hospital mortality was 2% in both groups (p = 1.00). No difference in net clinical adverse events or ischemic or bleeding complications was detected between the 2 groups. Bivalirudin reduced both ischemic and bleeding events in femoral-treated patients, but no such clinical benefit was observed in the radial-treated patients. In conclusion, as periprocedural PCI bleeding avoidance strategies have become paramount to optimize the clinical benefit, the interaction between bivalirudin and radial approach deserves additional investigation.
Compared to unfractionated heparin (UFH) with and without a platelet glycoprotein inhibitor (GPI), bivalirudin (Angiomax, Medicines Company, Parsippany, New Jersey), a direct thrombin inhibitor has been proved to be an effective anticoagulant in different clinical settings. The ease of administration and predictable dose response makes it an attractive anticoagulant for patients undergoing percutaneous coronary intervention (PCI). However, most studies have compared bivalirudin and heparin plus platelet glycoprotein IIb/IIIa receptor inhibitors (GPI). Furthermore, this evidence has been based on data related to the femoral approach, and data relating to the transradial route, which has been shown to have a better safety profile, remain sparse. Thus, we investigated the use and potential clinical benefit of bivalirudin compared to UFH in a large-volume radial center.
Methods
From April 2009 to February 2011, we identified all patients at our center who had been treated with bivalirudin during PCI. They were then matched to a similar group of patients with an identical access site and who had been treated with UFH during the same period. We excluded patients with an access crossover owing to a failure of procedure completion according to the initially planned strategy. The initial choice of access site was left to the operator. At our center, the radial or ulnar approach is used by default in all clinical scenarios.
All patients were pretreated with aspirin and clopidogrel before catheterization. As per our routine, all patients treated with the radial approach received an initial UFH bolus of 70 IU/kg after sheath insertion. At the discretion of the operator, an extra bolus dose of 30 IU/kg was given before PCI. The bolus dose was omitted if crossover to bivalirudin occurred or the patient was deemed to have a greater bleeding risk (i.e., those with a recent dose of low-molecular-weight heparin or fondaparinux, chronic anticoagulation, or planned concomitant use of a GPI). In the ST-segment elevation myocardial infarction population, UFH was administered to achieve an activated clotting time of >250 seconds. In patients undergoing the femoral approach, no UFH was given after sheath insertion. All patients treated with bivalirudin received a bolus dose of 0.75 mg/kg, followed by an infusion of 1.75 mg/kg/hour if the glomerular filtration rate was >30 ml/min/1.73 m 2 or 1.0 mg/kg/hour if the glomerular filtration rate was <30 ml/min/1.73 m 2 . The use of femoral closure devices (Angio-Seal, St. Jude Medical, St. Paul, Minnesota; or Perclose, Abbott Vascular, Abbott, Park, Illinois) was at the discretion of the operator.
Procedural success was defined as a final Thrombolysis In Myocardial Infarction flow of 3 with a residual stenosis of <30% and no major clinical complications. Death was defined as all-cause mortality. Periprocedural myocardial infarction was defined as creatinine kinase-MB elevation >3× the upper limit of normal after PCI (>30 IU in our laboratory). Target vessel revascularization was defined as ischemia-driven percutaneous or surgical revascularization of the treated vessel. Major bleeding was characterized by the previously defined Thrombolysis In Myocardial Infarction, major, and Bleeding Academic Research Consortium type 3 or 5 definitions. Major adverse cardiac events (MACE) were defined as death, myocardial infarction, and target vessel revascularization, and net adverse clinical events as MACE plus bleeding. The baseline bleeding risk was calculated using the Mehran bleeding score.
Categorical variables are expressed as numbers and percentages and continuous variables as the mean ± SD. The baseline and procedural characteristics were compared using Fischer’s exact test for categorical variables and Student’s t test or Wilcoxon/Kruskal-Wallis test for continuous variables. p Values <0.05 were considered significant. Statistical tests were performed using JMP, version 7.0 (SAS Institute, Cary, North Carolina).
Results
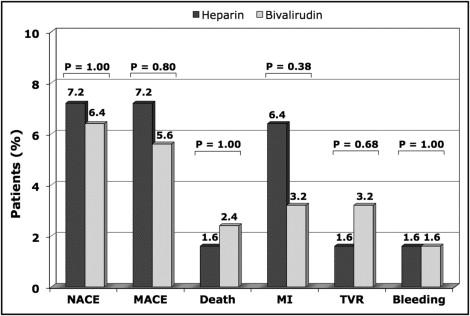
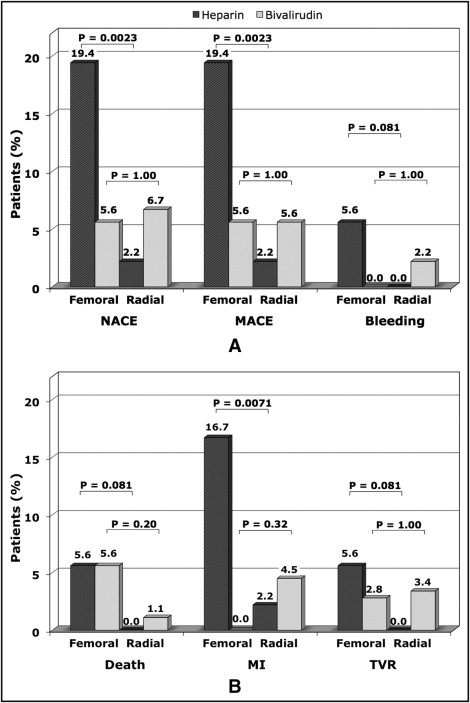
A total of 250 patients were studied during the study period ( Table 1 ). Patients in the bivalirudin group were older (72 ± 13 vs 66 ± 11 years, p <0.0001), more often had hypertension (76% vs 63%, p = 0.0388), had worse renal function (estimated glomerular filtration rate <60 ml/min, 51% vs 30%, p = 0.0012), and presented more often for primary PCI (30% vs 15%, p = 0.0037). Patients treated with bivalirudin more often had 6F sheaths (82% vs 64%, p = 0.0027) and concomitant use of low-molecular-weight heparin or fondaparinux before PCI (13% vs 1%, p = 0.0007, and 8% vs 0%, p = 0.0034, respectively). In contrast, the rate of GPI use was greater in the UFH group (20% vs 3%, p <0.0001). The patients in the 2 groups had a similar baseline Mehran bleeding score (14 ± 9 vs 15 ± 8, p = 0.48). The number and type of stents used, procedural duration, and use of a femoral closure device were similar in both groups ( Table 1 ). Overall, no significant differences in net clinical adverse events or ischemic or bleeding complications were observed between the 2 groups. In-hospital mortality was 2% in both groups (p = 1.00; Table 2 and Figure 1 ). In the UFH-treated patients, the radial approach was associated with a significant reduction in both bleeding and ischemic complications compared to the femoral approach; hence, it was associated with a significantly better clinical benefit (p = 0.0023). In the bivalirudin-treated patients, most of the benefit was observed in the femoral-treated patients, and no apparent benefit for bleeding or ischemic outcomes was noted in the radial-treated patients ( Figure 2 ).
| Variable | All (n = 250) | Heparin (n = 125) | Bivalirudin (n = 125) | p Value |
|---|---|---|---|---|
| Age (years) | 69 ± 12 | 66 ± 11 | 72 ± 13 | <0.0001 |
| Age ≥70 years | 128 (51%) | 50 (40%) | 78 (62%) | <0.0001 |
| Men | 163 (65%) | 81 (65%) | 82 (66%) | 1.00 |
| Diabetes mellitus | 89 (36%) | 41 (32%) | 48 (38%) | 0.428 |
| Treated dyslipidemia | 164 (66%) | 82 (66%) | 82 (66%) | 1.000 |
| Treated hypertension | 174 (70%) | 79 (63%) | 95 (76%) | 0.039 |
| Current smoker | 51 (20%) | 24 (19%) | 27 (22%) | 0.754 |
| Cardiogenic shock | 3 (1%) | 1 (1%) | 2 (2%) | 1.00 |
| Previous percutaneous coronary intervention | 61 (24%) | 31 (25%) | 30 (24%) | 1.00 |
| Previous coronary bypass surgery | 63 (25%) | 29 (23%) | 34 (27%) | 0.56 |
| Estimated glomerular filtration rate (ml/min) | 75 ± 38 | 82 ± 40 | 69 ± 35 | 0.003 |
| Estimated glomerular filtration rate <60 ml/min | 99 (40%) | 38 (30%) | 61 (51%) | 0.001 |
| Stable angina pectoris | 43 (17%) | 28 (22%) | 15 (12%) | 0.043 |
| Unstable angina pectoris | 71 (28%) | 40 (32%) | 31 (25%) | 0.262 |
| Non–ST-segment elevation myocardial infarction | 79 (32%) | 39 (31%) | 40 (32%) | 1.00 |
| ST-segment elevation myocardial infarction | 56 (22%) | 18 (14%) | 38 (30%) | 0.004 |
| Sheath size | ||||
| 5F | 61 (24%) | 40 (32%) | 21 (17%) | 0.008 |
| 6F | 182 (73%) | 80 (64%) | 102 (82%) | 0.003 |
| 7F | 7 (3%) | 5 (4%) | 2 (2%) | 0.447 |
| Procedural duration (min) | 63 ± 38 | 60 ± 36 | 66 ± 40 | 0.075 |
| Radial access | 178 (71%) | 89 (71%) | 89 (71%) | 1.00 |
| Average stents (n)/patient | 1.9 ± 1.2 | 1.7 ± 1.0 | 2.0 ± 1.4 | 0.083 |
| Left main artery | 26 (10%) | 8 (6%) | 18 (14%) | 0.06 |
| Left anterior descending artery | 108 (43%) | 45 (36%) | 63 (50%) | 0.029 |
| Left circumflex artery | 61 (24%) | 30 (24%) | 31 (25%) | 1.00 |
| Ramus intermedius | 9 (4%) | 5 (4%) | 4 (3%) | 1.00 |
| Right coronary artery | 87 (35%) | 50 (40%) | 37 (29%) | 0.11 |
| Saphenous vein graft | 22 (9%) | 11 (9%) | 11 (9%) | 1.00 |
| Procedural success | 226 (90%) | 115 (92%) | 111 (88%) | 0.52 |
| Femoral closure device | 49 (20%) | 23 (18%) | 26 (20%) | 0.75 |
| Heparin | 167 (67%) | 125 (100%) | 42 (34%) | <0.0001 |
| Low-molecular-weight heparin | 15 (6%) | 1 (1%) | 14 (13%) | 0.0007 |
| Fondaparinux | 9 (4%) | 0 | 9 (8%) | 0.003 |
| Warfarin | 2 (1%) | 0 | 2 (2%) | 0.498 |
| Glycoprotein IIb/IIIa inhibitors | 29 (12%) | 25 (20%) | 4 (3%) | <0.0001 |
| Variable | All (n = 250) | Heparin (n = 125) | Bivalirudin (n = 125) | p Value |
|---|---|---|---|---|
| Death | 5 (2%) | 2 (2%) | 3 (2%) | 1.00 |
| Myocardial infarction | 12 (5%) | 8 (6%) | 4 (3%) | 0.38 |
| Target vessel revascularization | 6 (2%) | 2 (2%) | 4 (3%) | 0.68 |
| Stroke | 0 | 0 | 0 | 1.00 |
| Unplanned revascularization | ||||
| Percutaneous coronary intervention | 1 (1%) | 0 | 1 (1%) | 1.00 |
| Emergent coronary bypass surgery | 1 (1%) | 1 (1%) | 0 | 1.00 |
| Stent thrombosis | 5 (2%) | 2 (2%) | 3 (2%) | 1.00 |
| Hemoglobin (g/L) | ||||
| Before | 128 ± 19 | 131 ± 19 | 126 ± 20 | 0.05 |
| After | 121 ± 19 | 129 ± 19 | 118 ± 20 | 0.02 |
| Change | 7 [6–9] | 8 [2–14] | 7 [0–13] | 0.40 |
| Anemia | ||||
| Baseline | 99 (40%) | 42 (34%) | 57 (46%) | 0.07 |
| After percutaneous coronary intervention | 141 (56%) | 62 (50%) | 79 (63%) | 0.04 |
| Troponin T >3 × upper limit of normal | 62 (32%) | 28 (27%) | 34 (40%) | 0.06 |
| Bleeding | ||||
| Retroperitoneal | 2 (1%) | 1 (1%) | 1 (1%) | 1.00 |
| Urinary | 1 (1%) | 0 | 1 (1%) | 1.00 |
| Access site, large hematoma | 1 (1%) | 1 (1%) | 0 | 1.00 |
| Blood transfusion | 6 (2%) | 3 (2%) | 3 (2%) | 1.00 |
| Blood transfusion ≥2 U | 4 (2%) | 1 (1%) | 3 (2%) | 0.62 |
| Thrombolysis In Myocardial Infarction, major | 3 (1%) | 1 (1%) | 2 (2%) | 1.00 |
| Bleeding Academic Research Consortium type 3–5 | 4 (2%) | 2 (2%) | 2 (2%) | 1.00 |
Details of the patients with bleeding or transfusion are summarized in Table 3 . Two patients in the UFH group had a complication requiring transfusion that was directly related to the initial femoral access site used. In contrast, no primary access site bleeding occurred in the bivalirudin group. In the bivalirudin group, 1 patient who had received aggressive anticoagulation and antiplatelet treatment had an access femoral site bleeding episode only after bilateral transfemoral puncture was required for intra-aortic balloon pump and hemodialysis access. Another patient experienced a major bleeding incident after accidently stepping on his Foley catheter. He had also been heavily anticoagulated with a therapeutic international normalized ratio of 2.3 for atrial fibrillation and had received fondaparinux before catheterization. The cause of in-hospital deaths is listed in Table 4 . In each group, 1 patient died from a direct complication from the procedure. Two patients in the bivalirudin group died after undergoing PCI as an emergency/bailout procedure.
| Pt. No. | Age (y) | Gender | Diabetes Mellitus | eGFR (ml/min) | Diagnosis | Coronary Artery Dilated | Antithrombotic Regimen (H/B/G/E/Fd/W) | Access Site (F/R) | Bleeding Site | Preprocedure Hemoglobin (g/L) | Lowest Hemoglobin (g/L) | PRBCs Transfused (U) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | Female | No | 119 | STEMI | Right | +/0/+/0/0/0 | +/0 | Retroperitoneal | 119 | 59 | 4 |
| 2 | 66 | Male | No | 80 | STEMI | Right | +/+/+/0/0/0 | +/+ | Retroperitoneal | 104 | 96 | 4 |
| 3 | 72 | Male | Yes | 60 | UA | SVG | +/0/0/+/0/0 | 0/+ | 0 | 96 | 83 | 1 |
| 4 | 81 | Female | Yes | 19 | NSTEMI | Cx | +/0/0/0/0/0 | +/0 | Femoral site | 82 | 73 | 1 |
| 5 | 82 | Male | No | 38 | STEMI | Right | 0/+/0/0/+/+ | 0/+ | Urinary | 132 | 85 | 2 |
| 6 | 84 | Male | No | 35 | STEMI | Right | +/+/0/0/0/0 | 0/+ | 0 | 95 | 84 | 2 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


