Fig. 15.1
Endoscopic view of Barrett’s esophagus epithelium. Endoscopic view of a long segment of columnar epithelium (salmon pink) above the gastroesophageal junction on a background of squamous epithelium (gray)
Detection and diagnosis of BE by white light endoscopy alone has a sensitivity from 80 to 90 % [23, 24]. Nodules, ulcers, and other mucosal abnormalities should have targeted biopsies because they are more likely to have dysplasia or cancer. Adjunctive techniques to increase the sensitivity of BE detection include autofluorescence, chromoendoscopy, magnification, and confocal endoscopy [25–27]. Although some of these modalities appear promising, none of these techniques have been shown to provide additional clinical information beyond high-resolution white light endoscopy to justify routine use in surveillance.
Endoscopic Ultrasound (EUS)
Before endoscopic therapy, EUS-guided fine-needle aspiration should be considered in select cases of HGD and IMC. This is based on a study that suggested EUS detection of unrecognized malignant lymphadenopathy changed management strategies in as many as 20 % of patients [28]. However, others do not use EUS in patients with flat mucosa and HGD on biopsy or do not feel it is necessary at all. EUS may be inaccurate and EMR is superior to EUS for local T staging [29].
Endotherapy
Endoscopic therapy has evolved as a safe and effective method of treating BE and IMC. Once identified, numerous endoscopic management options are available for patients with BE, based on the presence and grade of BE-associated dysplasia (Table 15.1). An important element to endoscopic therapy of dysplasia is PPIs given after endotherapy allow injured mucosa to heal and re-epithelization of new squamous mucosa.
Table 15.1
Barrett’s esophagus endoscopic management strategies
Histology | Intervention options |
|---|---|
NDBE | Consider no surveillance. |
If surveillance is elected, perform EGD every 3–5 years with 4-quadrant biopsies every 2 cm. | |
Consider endoscopic ablation in select cases. | |
IGD | Clarify presence and grade of dysplasia with expert Gl pathologist. |
Increase antisecretory therapy to eliminate esophageal inflammation. | |
Repeat EGD and biopsy to clarify dysplasia status. | |
LGD | Confirm with expert Gl pathologist. |
Repeat EGD in 6 months to confirm LGD. | |
Surveillance EGD every year, 4-quadrant biopsies every biopsies every 1–2 cm. | |
Consider endoscopic resection or ablation. | |
HGD | Confirm with expert GI pathologist. |
Consider surveillance EGD every 3 months in select patients, 4-quadrant biopsies every 1 cm. | |
Consider endoscopic resection or RFA ablation. | |
Consider EUS for local staging and lymphadenopathy. | |
Consider surgical consultation. |
Resection
EMR and ESD are techniques intended to remove superficial GI tissue (EMR) or large en bloc strips of mucosa (ESD). EMR is indicated for short-segment dysplastic BE, nodular dysplasia, and superficial (T1a) EAC. ESD can be used in similar situations but may be more preferred for extensive dysplastic lesions or IMC. EMR can be performed using a band ligator or cap. Both techniques have similar depth and complication profiles [30]. Multiband ligation is more efficient at removing a wider field, but the optimal technique should be determined by the preference of the endoscopist. During ESD, endoscopic tools are used to dissect lesions from the submucosa. ESD can often remove larger lesions intact than EMR, but expertise in this technique in the esophagus is not widely available in the United States. More information on various techniques used to perform EMR and ESD are described elsewhere [31].
Complications of EMR include bleeding, perforation, and stricture formation. Immediate bleeding may occur in 10 % of patients whereas delayed bleeding is rare [28, 32, 33]. Perforation is reported in less than 3–7 % of patients at high-volume centers [34–36]. Stricture formation occurs in 17–37 %, but rates may vary depending on circumference and length of mucosa removed by EMR [37]. However, when stepwise radical endoscopic resection is employed, stricture rates can be as high as 88 % have been reported. Most strictures can be managed by endoscopic dilation.
Unlike ablative techniques, EMR gives histopathologic information on depth and stage of the lesion (to estimate risk of lymph node metastases) and adequacy of resection. Therefore, EMR of nodular or dysplastic BE is often performed for diagnostic purposes before proceeding with ablative therapy, particularly for T1b dysplasia (which has an increased risk of lymph node metastases and failure of endoscopic therapy) [32].
Long-term outcomes of EMR for IMC demonstrated 95.7 % complete response rate at 5 years. EMR can be performed focally or for the entire BE epithelium. However, focal EMR alone is associated with high recurrence rates of 14–47 % [32, 38–44]. Complete eradication of BE epithelium is also known as circumferential EMR, stepwise radical endoscopic resection, or wide area EMR. This approach seeks to resect all known neoplasia as well as all at risk BE that may harbor potential synchronous and metachronous lesions. The response rates of circumferential EMR have ranged from 76 to 100 % in studies [33, 35, 37, 45]. Results of ESD for EAC showed 100 % en bloc resection rates and 80 % curative resection rates. In one study comparing EMR and ESD for large (>20 mm) esophageal squamous cell cancers, EMR had a higher local recurrence rate than ESD (23.9 % vs. 3.1 %) [46].
Ablation
Ablative techniques include photodynamic therapy (PDT), radiofrequency ablation (RFA), and cryotherapy. In the past, PDT was the primary ablative therapy for BE. PDT utilizes photosensitizer agents such as 5-aminolevulinic acid and porfimer sodium that produce a cytotoxic reaction after being stimulated by a certain wavelength of light in the presence of oxygen. Potential complications of PDT include bleeding, skin photosensitivity for as long as 1 month, and stricture formation in 30 % of patients [47, 48]. Another disadvantage of PDT is the high rate of buried metaplastic glands that harbor neoplastic potential and decreased efficacy when compared with newer modalities. Studies demonstrated initial and long-term success of PDT for eliminating HGD (77 % over 5 years) and early EAC [47]. Because of its side effect profile and inability to eliminate NDBE, PDT is less commonly used for dysplasia since the emergence of RFA.
RFA involves application of radiofrequency energy to esophageal mucosa with the HALO system (BARRX medical, Sunnyvale, California). The thermal energy is delivered by a balloon embedded with closely spaced electrodes. It is advantageous because it generates a uniform circumferential thermal injury with controlled depth, potentially explaining the lower rate of stenosis compared to EMR and low rate of buried metaplasia. Complications of RFA include noncardiac chest pain which generally subsides after 1 week [49]. Other potential complications include lacerations, bleeding, and stenosis (6 %). A multicenter sham-controlled trial using RFA for LGD and HGD demonstrated complete BE eradication in 90.5 % of patients with LGD and 81 % patients with HGD with lower rates of disease progression in the treatment arm compared with controls (3.6 % vs. 16.3 %) and fewer cancers (1.2 % vs. 9.3 %) [49]. Recently published data demonstrated eradication of dysplasia in 98 % and metaplasia in 91 % of patients at 3 years [50].
Cryotherapy involves cellular destruction of esophageal mucosa by freeze-thaw cycles. Cryotherapy utilizes a spray catheter being passed through a working channel of the endoscope, and either liquid nitrogen or carbon dioxide is applied to the dysplastic area. The spray is applied for a total of 40 s (two 20-s or four 10-s applications). Cryotherapy has a very good safety profile with a low rate of potential complications that include chest pain, bleeding, and strictures. One case of perforation has been reported. Cryotherapy also results in a low rate of buried metaplasia. A case series of 60 patients with BE and HGD demonstrated elimination of HGD in 97 %, all dysplasia in 87 %, and all BE in 57 % [51].
Adjunctive Techniques
Argon plasma coagulation (APC) is a modality that uses a noncontact electrocoagulation device with high-frequency monopolar current conducted via flow of ionized argon gas. Depth of tissue penetration may vary with factors such as generator power setting, gas flow, distance of probe from tissue, and duration of application [52]. Limited data support the usage of APC as a primary treatment modality [53, 54] of HGD and IMC. It is also limited by its nonuniform ablation of tissue, need for repeat sessions, and risk of persistent buried metaplastic glands. However, some experts have used APC as an adjunctive modality to ablate hard-to-reach areas of BE mucosa.
Hybrid Approaches
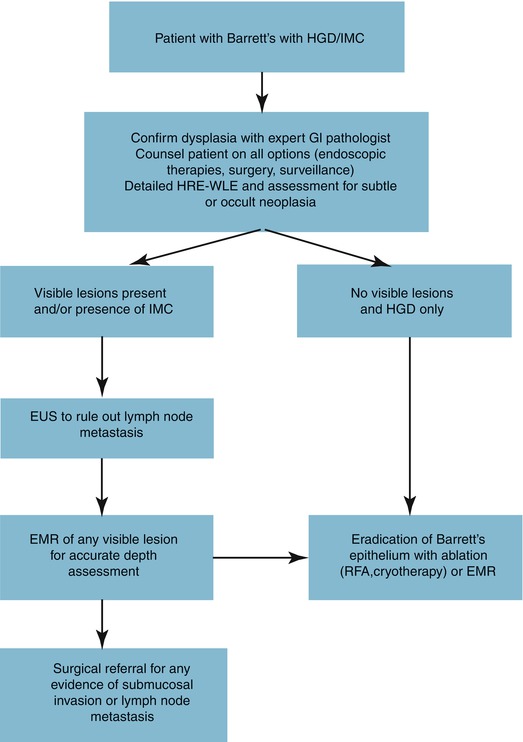
As previously mentioned, EMR for dysplasia and early EAC is safe in experienced hands with excellent 5-year survival. However, it may not be more beneficial than a hybrid approach, employing that EMR is used for all visible lesions and remainder of epithelium treated with serial RFA. This hybrid approach demonstrated complete response rates of neoplasia and metaplasia of 83–95 % and 79–88 %, respectively, in two trials [55, 56]. A randomized control trial compared stepwise radical endoscopic resection (focal EMR followed by serial EMR) versus a EMR/RFA approach. The two groups had similar complete remission rates (100 % vs. 96 %) but more stenosis in the radical resection group (88 % vs. 14 %) [57]. Thus, the hybrid approach is a good balance of efficacy and complication profile. A summary of how HGD or IMC is treated at our institution is shown in Fig. 15.2.
Indications for Esophagectomy
After endoscopic resection, positive deep margins of resection specimens and submucosal invasion of tumor are indications for esophagectomy. However, superficial submucosal tumors (have a lower rate of lymph node metastases than deeper submucosal lesions) have been treated successfully endoscopically [58], but further studies are warranted.
Esophagectomy is the only treatment modality for HGD that removes all neoplastic tissue, occult malignancy, and regional lymph nodes. However, it also has the highest rate of procedure-related mortality and morbidity. The mortality rates for esophagectomy have been shown to correlate inversely with the volume of the center performing it with rates ranging from 3 to 12 % [59, 60]. The risk of lymph node metastases for HGD and IMC has been reported as 0 and 1–2 %, respectively [61]. Esophagectomy involves removal of local lymph nodes and potentially does not guarantee cure for a tumor that has metastasized to lymph nodes. However, with endoscopic therapy, esophagectomy can often be avoided.
Patients that are more likely to benefit from esophagectomy are patients with submucosal invasion, evidence of lymph node metastasis, and failed endoscopic therapy or select high-risk patients with HGD/IMC (such as young patients with long-segment BE or multifocal dysplasia) [62].
Intense Surveillance
Intense surveillance involves endoscopic examinations of HGD every 3–6 months, withholding invasive treatments like esophagectomy until biopsy specimens reveal adenocarcinoma. Few studies support this approach, as various series have reported multiple patients with incurable disease (metastases) when the cancer was first detected on surveillance endoscopy while compliant with surveillance or when lost to follow-up [63–65]. Thus, we feel this approach should be discouraged in patients with HGD.
Conclusion
Although there are many unresolved issues regarding optimal management of BE, we follow guidelines put forth by medical societies such as the American Gastroenterological Association in 2011 and American Society of Gastrointestinal Endoscopy in 2012. Patients with BE should be treated with PPI and be considered for PPI even in the absence of GERD symptoms or reflux esophagitis endoscopically after risks and benefits are discussed with the patient.
We recommend patients with BE with no dysplasia, LGD, and HGD (in the absence of eradication therapy) have endoscopic surveillance as described. The diagnosis of dysplasia should be confirmed by an expert GI pathologist. The optimal treatment of HGD and IMC should take into account factors such as patient age, comorbidities, extent of BE/dysplasia, available expertise, and patient preferences. However, in most patients with BE-associated HGD, we recommend endoscopic eradication therapy rather than surgery or intensive surveillance. This involves EMR for removal and staging of visible lesions (if present) followed by RFA or PDT to ablate remaining metaplastic epithelium. Surgery is a reasonable alternative in young patients with HGD and long-segment BE or multifocal dysplasia, whereas intensive surveillance is reasonable in elderly and frail patients where endoscopic therapy might pose a substantial risk.
In the future, further studies will likely solidify the role of endoscopy in diagnosis, surveillance, and treatment of BE. More clinical trials, long-term data for the various treatment modalities and hybrid approaches, and studies on risk stratification and surveillance are still needed.
References
1.
2.
3.
Rugge M, Fassan M, Cavallini F, Zaninotto G. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2012;104(22):1771.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree