Fig. 9.1
The axillary artery is defined anatomically by the lateral margin of the first rib proximally and lateral edge of the teres major muscle distally. In its proximally protected location surrounded by chest wall, pectoral, and shoulder muscles, the axillary artery is protected from penetrating injury. The pectoralis minor muscle serves as the gateway to the midaxillary artery and is draped over the anterior surface of the vessel. Intimately associated with the third portion of the axillary artery are the major nerves to the arm, shoulder, and pectoral muscles
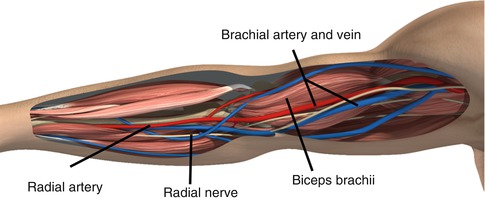
Fig. 9.2
The brachial artery runs with the median nerve along the anterior compartment of the arm. The profunda brachial branch joins the radial nerve and penetrates an intermuscular septum laterally becoming the radial collateral artery which is an important proximal collateral to the forearm following injury below the level of the mid-biceps
The axillary artery is defined anatomically by the lateral margin of the first rib proximally and lateral edge of the teres major muscle distally. In its proximally protected location surrounded by the chest wall and pectoral and shoulder muscles, the axillary artery is protected from penetrating injury. Due to its anatomical location, the axillary artery may present a formidable challenge to the operative surgeon seeking direct surgical exposure for proximal control. The remainder of the mid- and distal axillary artery becomes more superficial and easily accessible heading toward the axilla. The pectoralis minor muscle serves as the gateway to the midaxillary artery and is draped over the anterior surface of the vessel. Along the length of the artery from proximal to distal are an increasing number of branch arteries to the chest wall and shoulder. Intimately associated with the third portion of the axillary artery are the major nerves to the arm, shoulder, and pectoral muscles. This close relationship underscores the likelihood of combined neurovascular injuries with wounding as well as inherent challenges in rapid, efficient exposure and repair without causing unnecessary iatrogenic injury.
The brachial artery runs with the median nerve along the anterior compartment of the arm. The profunda brachial branch joins the radial nerve and penetrates an intermuscular septum laterally becoming the radial collateral artery which is an important proximal collateral to the forearm following injury below the level of the mid-biceps. This important collateral may support distal viability in the setting of major arterial injury and allow time for more serious associated injuries to be addressed. The superior ulnar collateral artery accompanies the ulnar nerve at mid-brachium and penetrates the intermuscular septum medially. The brachial artery is accompanied by two veins: the basilic which is superficial at the antecubital level and then penetrates the fascia to join the brachial vein and the brachial vein with its numerous deep and superficial anastomoses which unite at the level of the lateral border of the teres major to become the axillary vein.
A timeless adage still holds that standard vessel exposure should not be altered or compromised by traumatic wounds with the principles of proximal and distal control obtained whenever possible in the safest and most familiar manner. The most proximal axillary artery beneath the clavicle and adjacent to the first rib presents a major problem for hasty exposure such that a more traditional proximal mediastinal or intrathoracic subclavian exposure might be suitable. In a modern practice, these morbid exposures lend themselves more appropriately to the promise and benefit of rapid and less invasive endovascular control such as balloon occlusion from brachial or femoral access. The Seldinger technique is used to access the femoral artery with a steep left anterior oblique projection of the chest and opacification of the aortic arch to demonstrate the origins of the great vessels. Depending on laterality, the innominate or left subclavian may be cannulated with the appropriate preformed catheter and selective hydrophilic glide wire and advanced toward the site of injury. Next, an exchange made for a stiffer support wire and a long, large-diameter sheath to support the appropriate diameter compliant occlusion balloon. This balloon can be gently inflated under fluoroscopy with the use of a stopcock to maintain inflation and arrest inflow. In special circumstances, the use of a long sheath near the site of injury may also afford the option of covered stent placement to seal extravasation, close an arteriovenous fistula, cover a pseudoaneurysm, or permit coil embolization of bleeding branch vessels.
The remainder of the mid- and distal axillary artery is more classically exposed with an incision two fingerbreadths below mid-lateral clavicle. This separates the underlying pectoralis major fibers with the neurovascular bundle beneath the clavipectoral fascia. Wider arterial exposure may be facilitated by dividing the attachment between the pectoralis minor and the coracoid process. The more distal axillary artery can be approached with an axillary incision and retraction of the lateral pectoralis border medially and coracobrachialis superiorly. In this position, the median nerve is encountered and needs to be gently mobilized. Being a superficial artery in the brachium lends the brachial artery to an easy exposure along the medial groove between the biceps and the triceps. The basilic vein, brachial vein, and median nerve must be identified and protected particularly during a hasty exposure to control bleeding.
9.3 Treatment Algorithm
The first decision confronting the attending surgeon is immediate surgery versus delay for additional imaging. For high-velocity blunt trauma and particularly with proximal upper extremity injury, there is a significant concurrence of associated head and neck, intrathoracic, and abdominal injury that may supersede extremity care. Following the basic tenets of advanced trauma life support protocols, resuscitation and triage and after addressing immediate life-threatening injuries affecting airway, breathing and circulation, attention is turned to the injured extremity. The first priority is life-sustaining hemorrhage control, the second goal relieving ischemia, and lastly stabilizing measures to mitigate future disability with a goal of restoring meaningful limb function beyond basic limb salvage. This includes orthopedic stabilization and repair, nerve repair and adequate soft tissue coverage combined with appropriate antibiotic usage, and a comprehensive and compulsive wound care plan with early and aggressive physical and occupational therapy.
From a purely vascular standpoint, the presenting symptoms of upper extremity vascular injury dictate triage and are divided into hard signs that mandate immediate exploration and softer signs that may beg additional investigation for diagnosis and prognosis and even afford the option of less invasive endovascular intervention or selective nonsurgical observation. The well-known hard signs include active hemorrhage, expanding hematoma, pulseless extremity, or frank ischemia as manifest clinically by pain, pallor, paresthesia, paralysis, poikilothermy, and pulselessness. These patients manifesting hard signs are prepared and taken immediately to the operating room for proximal and distal control of the injury site and exploration and repair of the artery. For the most proximal injuries at the clavicle level and a relatively stable patient, it is expected that intraoperative angiography with endovascular means for proximal control of bleeding through balloon occlusion with an option for definitive endovascular repair should be in the armamentarium of a modern vascular surgeon or collaborating interventional radiologist. The large diameter and fixed location of the subclavian-axillary vessels and their privileged location that challenges safe exposure make this area fruitful for endovascular endeavors and quite distinct from the more superficial and smaller-diameter arterial tree beyond the mobile shoulder and elbow joints.
A more stable patient without frankly ischemic signs may demonstrate softer signs of vascular injury such as an ominous mechanism or proximity of the wound to a major artery, large non-expanding hematoma, high-velocity missile, abnormalities in noninvasive perfusion measurement, bruit or thrill, suspicious mechanism with additional associated factors such as a major fracture or soft tissue injury that affects accuracy of serial examination or multiple sites of injury such as a shotgun blast that raises a high probability or suspicion with uncertainty about the location of the most critical vascular lesion. While traditional contrast-enhanced angiography has been the gold standard and affords opportunity for intervention with coil embolization and covered stent placement or at a minimum proximal control with endoluminal balloon occlusion, the ability to rapidly acquire high-specificity diagnostic imaging with spiral CT angiography has been a game changer as a rapid screening test and plays a major role in the management of multi-trauma patients. In particular, high-resolution diagnostic CT angiography is very useful when there are additional complex orthopedic and soft tissue injuries to be evaluated outside the limited view of angiography which only shows intraluminal blood flow and vessel wall contour. CT angiography also allows grading of injury and thereby allows triage decisions in addition to planning revascularization with conduit assessment and required exposure (Fig. 9.3).
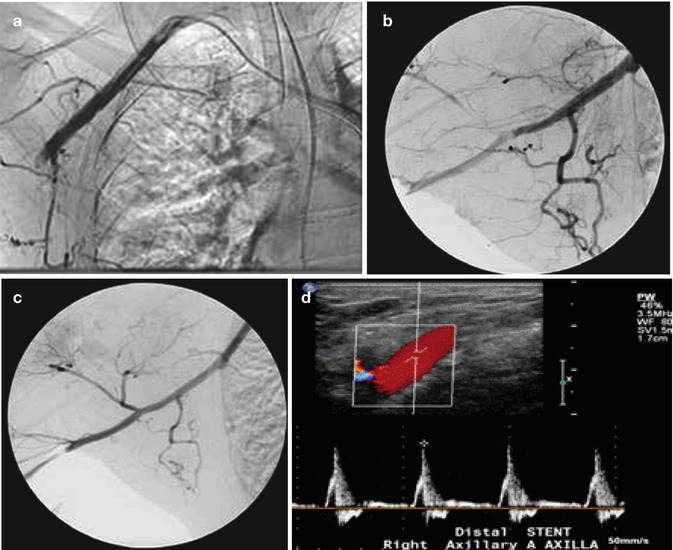





Fig. 9.3
(a) A 46-year-old male pedestrian struck with skull fracture, subarachnoid bleed, shoulder dislocation, lumbar fracture, and open tibia-fibula fracture. The hand ischemic in ICU. (b) Follow-up images after placement of 6 × 40 self-expanding stent. (c) A 52-year-old male fell 5 ft onto outstretched arm with shoulder dislocation. Unable to pass guidewire due to extrinsic compression. Treated successfully with vein graft from axillary artery to brachial artery (Courtesy of Dr. Hao Wu). (d) Duplex images of stent at 1-year post procedure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


