AV Access (Salvage— Endovascular)
LUKE R. WILKINS and WAEL E. SAAD
Presentation
A 72-year-old woman with end-stage renal disease (ESRD) on hemodialysis (HD) via a left upper extremity brachial-basilic arteriovenous graft presents with a pulsatile graft and a marked decrease in flow rates during most recent dialysis session.
Differential Diagnosis
The differential diagnosis of pulsatile graft with decrease flow rates on HD includes venous outflow stenosis occurring at the venous anastomosis or within the central venous system. Alternatively, an arterial inflow lesion can also cause decreasing flow rates and diminished thrill within the graft.
Workup
A complete medical and surgical history was obtained. The left upper extremity graft was placed approximately 2 years prior to presentation. In addition, the patient previously had a left-sided, tunneled hemodialysis (HD) catheter with access in the left internal jugular vein. After placement of the graft in the left upper extremity, the patient has had multiple prior interventions for recurrent central stenosis of the left brachiocephalic vein. At the most recent intervention, 2 months prior to presentation, the central stenosis required placement of a 20 mm × 40 mm Wallstent that was postdilated with a 16 mm × 4 cm Maxi LD balloon. The patient tolerated dialysis well until presentation when, at scheduled dialysis, a marked decrease in flow rates was identified (less than 400 mL/min). The patient reported no abnormal symptoms during most recent dialysis session. Pertinent negatives include no evidence of steal symptoms and no complaints of upper extremity swelling.
On physical examination, there is a pulsatile graft in the left upper extremity. No definite thrill could be identified. Brachial, ulnar, and radial pulses are palpable. A bedside ultrasound was performed that showed a patent arterial anastomosis with no evidence of stenosis or narrowing (Fig. 1). The venous outflow was also evaluated and showed no evidence of narrowing or stenosis.
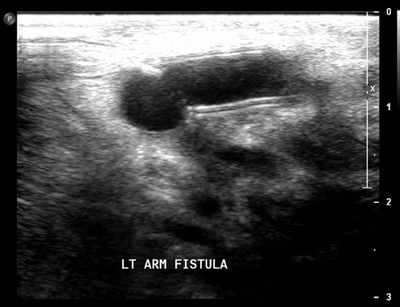
FIGURE 1 Ultrasound image at level of arterial anastomosis showing no evidence of stenosis or narrowing.
On the basis of the ultrasound, the cause for decrease in flow rates is likely secondary to either venous outflow obstruction or central venous disease (CVD). A digital subtraction central venogram was performed via antegrade access in the venous outflow. Images demonstrate previously placed Wallstent in the left brachiocephalic vein with marked degree of narrowing/stenosis at its central-most aspect with extensive collateral vessel formation (Fig. 2).
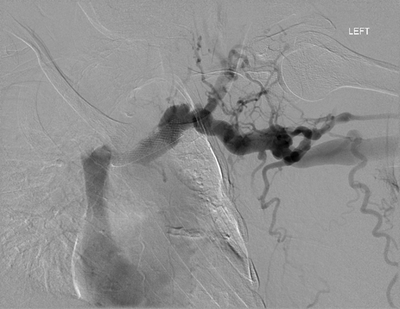
FIGURE 2 Central venogram via left upper extremity fistula showing Wallstent in left brachiocephalic vein with recurrent stenosis and marked collateral vessel formation.
Diagnosis and Treatment
The diagnosis of CVD may be made on the basis of several clinical and imaging findings. The provided clinical history of prior HD catheter and prior intervention should raise suspicion. Frequently, patients will present with ipsilateral face, neck, breast, or arm edema. In addition, the presence of massive arm edema in the access arm is virtually diagnostic of CVD. However, as can be seen in the provided case, the absence of significant swelling does not preclude the diagnosis. The presence of enlarged collateral veins in the neck, chest, and ipsilateral extremity may also be identified. The diagnosis of CVD may be suggested through findings on duplex ultrasound as absence of normal respiratory variation in the diameter of the central veins and polyphasic atrial waveforms. As demonstrated in the provided case, digital subtraction central contrast venography is the gold standard for the diagnosis of CVD.
Endovascular intervention is the primary treatment consideration for CVD. Current options include percutaneous transluminal angioplasty (PTA), bare-metal stent (BMS) placement, or covered stent (CS) placement. Currently, the Kidney Disease Outcome Quality Initiative (KDOQI) guidelines recommend PTA with or without stent placement as the preferred treatment option for CVD.
PTA is the traditional first-line therapy for CVD. However, most research involving PTA of CVD has lacked clearly defined reporting standards leading to highly variable methodologies and inconsistent conclusions. Generally, PTA has a high technical success with highly variable primary patency rates of 23% to 55% at 6 months and 12% to 50% at 12 months. Multiple repeated interventions with close follow-up are necessary when attempting to maintain patency in CVD patients with PTA.
Given efficacy of PTA, a more long-term, durable solution was sought. For this role, BMS have been used for maintenance of patency in CVD. Initial attempts focused on elastic or highly resistant fibrotic lesions. This approach is associated with a high rate of technical success (close to 100%). Primarily, self-expanding stents are utilized in this anatomical region. Balloon-expandable stents are not routinely used as they may deform or migrate. This is particularly evident in the subclavian and left brachiocephalic veins where adjacent osseous structures combined with respiratory motion may cause significant external compression. The first generation of self-expanding stents consisted of the Wallstent (Boston Scientific, Natick, MA). The advantages of this stent are its variability of diameter and lengths combined with its marked radiopacity. Second-generation stents are the nitinol stents. Nitinol is an alloy of nickel and titanium. This material will have a reversible shape transformation at high temperatures (martensitic transformation) that is preset by its nickel and titanium ratio.
While initial technical success is high for BMS in CVD, long-term results remain variable. Primary patency rates range from 63% to 100% at 3 months, 42% to 89% at 6 months, and 14% to 73% at 12 months. Cumulative patency results have ranged 72% to 100% at 3 months, 55% to 100% at 6 months, and 31% to 97% at 12 months. While the current literature demonstrates variable long-term patency results, there is clearly a significant population of CVD patients whose lesions are inadequately treated with PTA and will require BMS for both technical success and better long-term patency.
CS have recently been utilized with varying degrees of success in the treatment of CVD. In theory, CS provide a relatively inert and stable intravascular matrix for endothelialization while providing the mechanical advantages of a BMS. This could decrease the intimal hyperplastic response and reduce the restenosis rate seen after PTA or BMS. However, as with studies evaluating PTA and BMS, the results are varied with nonuniform methodology. Within these limitations, there may be a trend toward improved 3-, 6-, and 12-month patency after CS stent placement. In addition, the cost-benefit analysis of CS placement will need to be studied with long-term follow-up and comparative assessments to delineate the future role of this treatment option.
Lastly, while endovascular treatment is the first-line therapy for CVD, some patients may be refractory to endovascular options. In this patient population, surgical treatments may be the only option. While 12-month patency rates of open surgical management of CVD range from 79% to 86%, the procedure is frequently associated with significant morbidity. A full and complete discussion regarding surgical management of CVD is beyond the scope of this chapter, and the reader is referred to a dedicated text for a more detailed analysis.
Technical Approach
A 0.035 guidewire and angled tip catheter were used to cross the central venous stenosis. Given previously placed stent, great care was taken to ensure that the wire stayed in an intraluminal location without crossing the interstices of the stent (Figs. 3 and 4). Sequential balloon inflations were then performed at the area of stenosis with 6-, 8-, 10-, and 12-mm angioplasty balloons. Despite multiple, prolonged inflations, a marked degree of residual stenosis remained. At this point, the decision was made to extend the left brachiocephalic stent. While in this anatomic location a self-expanding stent is the preferred stent, given the location of the residual stenosis, precise deployment in the left brachiocephalic vein at the junction of the SVC was felt to be of utmost importance. Placement of a self-expanding stent in this location and with this type of lesion may result in stent migration. Further, the stenotic lesion was in the central-most aspect of the left brachiocephalic vein and posterior to the sternum, thus decreasing the risk for significant compressive forces related to respiratory motion. With these considerations in mind, the decision was made to place a balloon-expandable stent. A 12-French, 40-cm sheath was then advanced through the stent. A 10 × 39-mm Palmaz stent (Cordis, Miami Lakes, FL) was loaded on a 14 × 40 angioplasty balloon. The stent was then deployed within the distal left brachiocephalic vein stent extending just to the junction with the SVC. Repeat fistulogram was performed demonstrating markedly improved flow through the brachiocephalic vein without any filling of the previously noted collaterals along the left chest wall and left upper extremity (Fig. 5). The sheath was removed and a 2-0 purse string suture was then placed at the puncture site to achieve hemostasis Table 1.
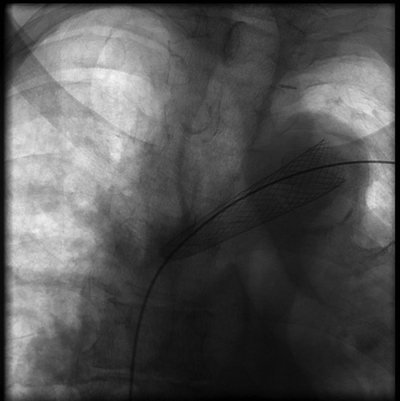
FIGURE 3 Image demonstrating wire access through existing Wallstent in the left brachiocephalic vein.



