Fig. 17.1
Polar maps of myocardial retention of C-11 metahydroxyephedrine ([11C]-mHED) in four cardiac transplant recipients at different times after surgery, illustrating time course and regional extent of sympathetic reinnervation (Reprinted with permission by Springer Science and Business Media, from Bengel and Schwaiger (2004))
Serial assessment using two [11C]-mHED PET scans within 3–4 years demonstrated a continuous increase of extent and intensity of reinnervation with time after transplantation (Bengel et al. 1999). Sympathetic nerve terminals first reappeared in the basal parts of the myocardium and then extended further into distal parts, while the apex was occasionally involved late after transplantation. This finding is consistent with growth of sympathetic fibers along arterial structures. If reinnervation occurs, basal parts are reached first. In addition to a gradient from base to apex, anterior and septal walls were reinnervated earlier, while the lateral wall was involved later. These results suggest that sympathetic nerves are first restored in the territory of the left anterior descending artery, while later the left circumflex territory is involved additionally. Complete restoration of sympathetic innervation, however, was not observed until 15 years after transplantation, because inferior myocardium consistently remained denervated (Bengel et al. 1999; Uberfuhr et al. 2000b).
Using regionally heterogeneous reinnervation as a model, several studies have validated the results of catecholamine imaging by comparison with alternative tests of sympathetic innervation. [11C]-mHED PET-derived evidence of ventricular reinnervation, e.g., correlated with invasive measurements of tyramine-induced coronary arteriovenous norepinephrine spillover (Odaka et al. 2001) and with electrophysiologic indexes of reinnervation derived from heart rate variability measurements (Ziegler et al. 1996; Uberfuhr et al. 2000a).
While time after surgery is considered a major determinant of presence and extent of reinnervation, observations of interindividual heterogeneity and the regionally incomplete pattern suggest that additional determinants are involved. This has been studied in a comparably large sample of 77 transplant recipients by a multivariate analysis. In this analysis using [11C]-mHED PET, some patients remained denervated until late after transplantation, and other factors such as donor and recipient age, duration and complexity of transplant surgery, and frequency of allograft rejection were identified as independent determinants of sympathetic reinnervation (Bengel et al. 2002) (Table 17.1). Aging has been suggested to be associated with reduced availability of target-derived neurotrophic factors, which may explain reduced sympathetic reinnervation with increasing age. Reduced availability and synthesizing capacity of neurotrophins in the myocardium may also explain the lower degree of reinnervation in case of more frequent rejection episodes. Also, because surgical dissection results in axonal degeneration, sympathetic nerve fibers need to regrow along arterial structures to reach the allograft as their target organ. Extensive areas of scar tissue or other morphologic alterations along the path of regrowth may thus impair reappearance of nerve terminals in the myocardium. This is confirmed by less extensive reinnervation in patients with aortic complications at transplant surgery and by a significant inverse correlation with aortic cross-clamp time (Bengel et al. 2002). Hence, the surgical procedure appears to be another factor which may influence reinnervation. The observation of more intense reinnervation in patients transplanted for dilated compared to ischemic cardiomyopathy (Bengel et al. 2002) may also be explained in this context, as regrowth along sclerotic aorta and other vessels may be more difficult.
Table 17.1
Parameters tested for association with cardiac transplant reinnervation
Recipient related | Donor related | Surgery related | Immunogenetical |
|---|---|---|---|
Time after HTX a | Age a,b | Allograft cold ischemia | Recipient gender |
Weight at HTX | Age difference | Donor gender | |
Height at HTX | Weight | Aortic cross-clamp time a,b | Gender mismatch |
Age at HTX a | Height | Rhesus mismatch | |
Body mass at HTX | Body mass | Perioperative aortic complications a | HLA A mismatch |
Ejection fraction prior to HTX | Body mass difference | HLA B mismatch | |
HLA DR mismatch | |||
Disease type a | CMV infection | Overall HLA mismatch | |
Duration of disease | Type of immunosuppression | ||
CMV infection | |||
Outcome after HTX | Rejection frequency a,b |
Finally, diabetes mellitus has also been shown to influence sympathetic reinnervation of the transplanted heart (Bengel et al. 2006). The regional extent of reinnervation and the regeneration rate were significantly reduced in diabetic transplant recipients compared to a matched transplant recipient group without diabetes (Fig. 17.2). The regenerative capacity of the sympathetic nervous system of the heart was reduced, but not abolished, by diabetes mellitus.
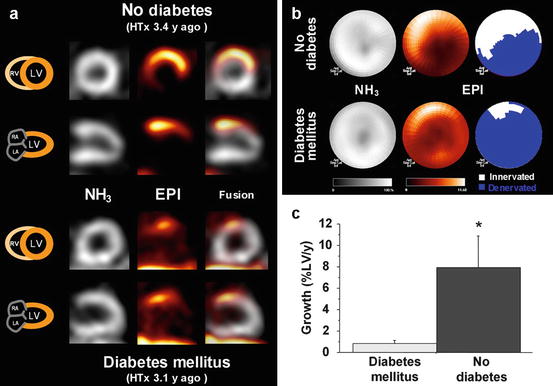

Fig. 17.2
Effect of diabetes mellitus on transplant reinnervation. Shown are representative left ventricular short- and long-axis tomographic images (a) and polar maps (b) of cardiac transplant recipient without evidence of diabetes mellitus (top) and another recipient with history of diabetes (bottom). Gray-scale images show homogeneous myocardial perfusion, determined by [13N]NH3. Color-scale images show regional uptake of the neurotransmitter [11C]-epinephrine, indicating reinnervation in basal anterior wall. Extent of reinnervation was 42 % in nondiabetic recipient and 13 % in diabetic recipient. (c) Group results (mean ± SE) for neuronal regeneration rate. EPI [11C]-epinephrine, HTx heart transplantation, LA left atrium, LV left ventricle, RA right atrium, RV right ventricle. *P < 0.05 (Reprinted with permission from Bengel et al. (2006). This research was originally published in JNM. © by the Society of Nuclear Medicine and Molecular Imaging, Inc)
17.4 Transplant Reinnervation: Functional Effects
The pattern of regionally heterogeneous reinnervation on the one hand makes the transplanted heart a good model to determine physiologic effects of sympathetic innervation in vivo, by an intraindividual comparison of innervated and denervated myocardium. On the other hand, it also raises the general question whether this regenerative process, which remains incomplete, has general beneficial functional effects for the transplant recipients. Both issues have been studied in various elegant multi-tracer radionuclide imaging studies.
PET was used to determine myocardial blood flow, flow response to the cold pressor test as an index of endothelial-dependent vasodilatation, and flow response to adenosine as a composite index of endothelial-dependent and endothelial-independent vasodilatation in non-rejecting, otherwise healthy reinnervated transplant recipients. They observed a significant improvement of flow response to cold pressor in innervated compared to denervated vascular territories, while there was no difference for the response to adenosine. These results demonstrated the importance of sympathetic innervation for regulation of endothelial-dependent vascular reactivity in general, and they also supported the physiologic relevance of reinnervation for transplant recipients (Di Carli et al. 1997). Other studies focused on the effect of innervation on myocardial substrate utilization: Higher utilization of glucose were found at equal rates of overall oxidative metabolism in denervated compared to reinnervated myocardium of allografts, suggesting a metabolic switch from free fatty acids to glucose under conditions of denervation (Bengel et al. 2000). In another study, non-invasively determined allograft efficiency was shown to be improved in transplant recipients compared with failing hearts and was comparable to normal hearts. Differences between denervated and reinnervated allografts were not surveyed, and the dependency on loading conditions and contractility was preserved. These data suggested that normal regulatory interactions for efficiency are intact and that sympathetic tone does not play a role under resting conditions (Bengel et al. 2001b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


