Drug class/generic name (brand)
Cardiac adverse events
Relative frequency of adverse effecta
Relative frequency of therapeutic use
Anthracyclines/anthraquinones
Doxorubicin (Adriamycin)
CHF/LV dysfunction
Common
Very frequent
Daunorubicin (Cerubidine)
CHF/LV dysfunction
Common
Very frequent
Epirubicin (Ellence, Pharmorubicin)
CHF/LV dysfunction
Common
Very frequent
Idarubicin (Idamycin)
CHF/LV dysfunction
Common
Very frequent
Mitoxantrone (Novantrone)
CHF/LV dysfunction
Uncommon
Infrequent
Alkylating agents
Busulfan (Myleran)
Endomyocardial fibrosis
Rare
Infrequent
Cardiac tamponade
Rare
Cisplatin (Platinol)
Ischemia
Uncommon
Very frequent
Hypertension
Frequent
CHF
Uncommon
Cyclophosphamide (Cytoxan)
Pericarditis/myocarditis
Rare
Very frequent
CHF
Uncommon
Ifosfamide (Ifex)
CHF
Uncommon
Common
Arrhythmias
Uncommon
Mitomycin (Mutamycin)
CHF
Uncommon
Infrequent
Antimetabolites
Capecitabine (Xeloda)
Ischemia
Rare
Very frequent
Cytarabine, Ara-C (Cytosar)
Pericarditis
Rare
Very frequent
CHF
Rare
Fluorouracil (Adrucil)
Ischemia
Uncommon
Very frequent
Cardiogenic shock
Rare
Antimicrotubules
Paclitaxel (Taxol)
Sinus bradycardia, AV block
Rare
Very frequent
Ventricular tachycardia
Rare
Hypotension
Rare
CHF
Uncommon
Vinca alkaloids
Ischemia
Uncommon
Common
Biological agents
Monoclonal antibodies
Alemtuzumab (Campath)
Hypotension
Common
Infrequent
CHF
Rare
Bevacizumab (Avastin)
Hypertension
Common
Common
CHF
Uncommon
Deep venous thrombosis
Rare
Cetuximab (Erbitux)
Hypotension
Rare
Common
Rituximab (Rituxan)
Hypotension, angioedema
Uncommon
Common
Arrhythmias
uncommon
Trastuzumab (Herceptin)
CHF/LV dysfunction
uncommon
Common
Interleukins
IL-2
Hypotension
Frequent
Infrequent
Arrhythmias
Uncommon
Denileukin diftitox (Ontak)
Hypotension
Frequent
Infrequent
Interferon-α
Hypotension
Common
Very frequent
Ischemia
Uncommon
LV dysfunction
Rare
Miscellaneous
All-trans retinoic acid (tretinoin)
CHF
Uncommon
Infrequent
Hypotension
Uncommon
Pericardial effusion
Rare
Arsenic trioxide (Trisenox)
QT prolongation
Frequent
Infrequent
Imatinib (Gleevec)
Pericardial effusion
Uncommon
Very frequent
CHF, edema
Common
Pentostatin (Nipent)
CHF
Uncommon
Infrequent
Thalidomide (Thalomid)
Edema
Uncommon
Infrequent
Hypotension
Rare
Deep venous thrombosis
Uncommon
Bradycardia
Uncommon
Etoposide (Vepesid)
Hypotension
Uncommon
Common
Anthracyclines, such as doxorubicin, are most commonly associated with cardiotoxicity. The anticancer effects of anthracyclines are mediated primarily through inhibition of DNA synthesis, transcription, and replication (Smith et al. 2010). Simultaneously, oxygen-derived free radicals are formed, causing direct damage to proteins, lipids, and DNA, which may lead to myocyte apoptosis in the heart. This process is thought to be the key mechanism in anthracycline cardiotoxicity. Alternate hypotheses include calcium overload of myocytes, alterations in adrenergic function, and inhibition of protein synthesis (Panjrath and Jain 2006). A meta-analysis of 55 randomized controlled trials demonstrated an increased risk of clinical cardiotoxicity by anthracycline-based regimens by 5.43 fold, subclinical cardiotoxicity by 6.25 fold, and cardiac death by 4.94 fold compared with non-anthracycline regimens (Smith et al. 2010). Risk factors for developing cardiotoxicity comprise a high cumulative anthracycline dose, mediastinal radiation therapy, combination chemotherapy, combination chemotherapy with monoclonal antibody therapy, preexisting cardiovascular disease, emphysema, diabetes, female sex, and very young or older age (Panjrath and Jain 2006; Yeh et al. 2004). Toxic effects on the myocardium due to anthracyclines may occur early, during or immediately after infusion, or late, after months up to 20 years after therapy. Toxic effects on the myocardium during or immediately after infusion are usually self-limiting with discontinuation. Chronic effects may persist after discontinuation of therapy, or may present after months or years, and can progress to overt cardiac dysfunction or CHF. Therefore, early detection before irreversible functional cardiac impairment has occurred is crucial.
[123I]-MIBG scintigraphy was investigated as an early marker for cardiotoxicity after anthracycline therapy. In a rat model, a clear dose-dependent reduction in MIBG uptake was demonstrated after doxorubicin therapy (Wakasugi et al. 1992). The reduction in MIBG uptake was larger and more linear dose-dependent than impairment of LVEF. Furthermore, MIBG uptake was significantly reduced after 6 weeks of therapy with only mild myocyte damage at histopathological examination, whereas LVEF decreased only slightly after 7 weeks and showed a remarkable decrease after 8 weeks of therapy, suggesting MIBG to be a sensitive biomarker for early detection of doxorubicin-induced cardiomyopathy (Wakasugi et al. 1993). The subendocardial layer appeared to be more vulnerable to doxorubicin than the subepicardium (Jeon et al. 2000). Congestive heart failure due to Adriamycin in an early stage in a rat model was found to accelerate exocytotic release of norepinephrine from cardiac adrenergic neurons, rather than disturb the neuronal uptake function (Wakasugi et al. 1995). In an advanced stage, nonexocytotic metabolic release is induced due to energy depletion, increasing norepinephrine release. Both mechanisms lead to a reduction of myocardial MIBG uptake.
In a case report, [123I]-MIBG scintigraphy with SPECT of a patient after doxorubicin therapy without cardiac symptoms and normal LVEF was described, showing reduced uptake in several segments and a high washout rate (WR) (Takano et al. 1995). After 10 months the patient died of CHF, and at necropsy structural changes, corresponding to the impaired segments at the [123I]-MIBG, were found in a dilated left ventricle (Figs. 23.1 and 23.2). Conversely, in segments with preserved [123I]-MIBG uptake, myocardial nerve fibers had a normal aspect.
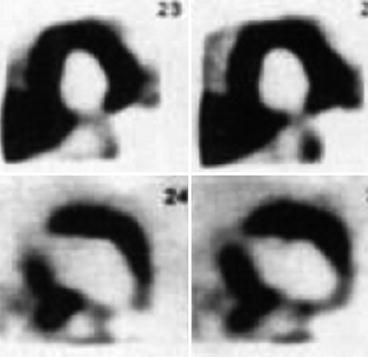
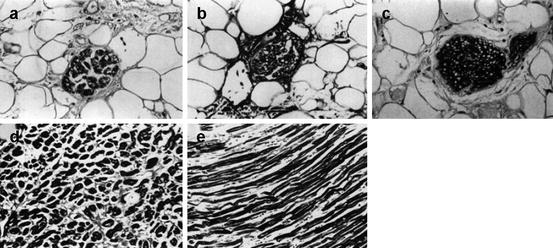

Fig. 23.1
A 52-year-old female patient, treated with doxorubicin for malignant lymphoma, had no cardiac symptoms, and a LVEF of 52 % was obtained by echocardiography. Illustration of [123I]-MIBG scintigraphy, delayed images, performed 2 weeks after the last chemotherapy treatment. [123I]-MIBG uptake was markedly reduced in the apical anterior, inferior, and lateral walls of the left ventricle. Delayed H/M ratio was reported to be 1.12 % with a WR of 49.5 %. 201Thallium uptake was normal in all segments, indicating normal cardiac perfusion (Reproduced with permission from Takano et al. (1995))

Fig. 23.2
Histology of the left ventricle after the patient had died from doxorubicin-induced cardiomyopathy 10 months after the performance of [123I]-MIBG scintigraphy. (a) S-100 stained section of the inferior wall, showing markedly atrophic nerve fibers. Prior [123I]-MIBG uptake herein was clearly reduced. (b) Azan-stained section of the inferior wall, showing atrophic and fibrotic nerve fibers. (c) S-100 stained section of the septum, depicting normal nerve fibers. The septum displayed normal [123I]-MIBG uptake. (d) Azan-stained section of the inferior wall, confirming atrophic myocytes and remarkable interstitial fibrosis, whereas the septum revealed a normal myocyte structure (e) (Reproduced with permission from Takano et al. (1995))
In patients receiving doxorubicin therapy, the dose-dependent reduction in [123I]-MIBG uptake (heart/mediastinum ratio (H/M ratio)) was confirmed in several studies (Lekakis et al. 1996; Takano et al. 1996; Takeishi et al. 1994; Valdes Olmos et al. 1995). Even at low dosage, the H/M ratio showed a decline, whereas LVEF derived by echocardiography or multi-gated radionuclide ventriculography was preserved. The 4-h WR tended to increase but did not reach statistical significance in a small, proof-of-concept study (Valdes Olmos et al. 1995). Complementary SPECT examinations to identify segment abnormalities were of good quality in patients with normal H/M ratio, though 4-h SPECT images of patients with abnormal tracer retention were of poorer quality and needed a longer acquisition time.
When [123I]-MIBG scintigraphy was compared with [111In]-antimyosin scintigraphy in the evaluation of cardiotoxicity after doxorubicin therapy, [111In]-antimyosin detected early myocardial damage at intermediate doses of doxorubicin, whereas H/M ratio was only impaired at high cumulative doses (Carrio et al. 1995). In four patients with symptomatic anthracycline-induced cardiomyopathy, [123I]-MIBG and [111In]-antimyosin scintigraphy was performed 2–116 months after the onset of CHF, showing a persistent decreased H/M ratio in [123I]-MIBG and an increased heart-to-lung ratio (H/L ratio) in [111In]-antimyosin scintigraphy in all patients, suggesting permanent damage to the cardiac adrenergic nerve endings and myocytes (Nousiainen et al. 2001). H/M and heart/lung (H/L) ratios remained impaired even in patients who had recovered clinically, and LVEF had returned to near normal.
All of these studies included only a limited number of patients and lacked appropriate follow-up. No studies have been performed yet for other chemotherapeutic agents, such as alkylating agents or antimetabolites, using [123I]-MIBG scintigraphy as a biomarker to evaluate cardiotoxicity.
23.2.2 Effects of Monoclonal Antibody Therapy
Several monoclonal antibody therapeutic agents may have cardiovascular side effects, such as hyper- and hypotension, CHF, and arrhythmias (Table 23.1). Trastuzumab is a humanized monoclonal antibody against human epidermal growth factor receptor type 2 (HER2), which may be overexpressed in several cancer types and is at present frequently used as targeted therapy for breast cancer and esophagogastric cancer.
Trastuzumab is known to cause a (mostly) transient, asymptomatic decline in LVEF, but patients may develop CHF years after therapy (Di Cosimo 2011). Patients receiving a combination chemotherapeutic regimen including trastuzumab are at highest risk to present a cardiac event, CHF, or cardiac death (Russell et al. 2010; Tan-Chiu et al. 2005). The mechanism of trastuzumab-related cardiac toxicity is still largely unclear, though it is now considered a dual-step process. First, the expression of HER2 is increased or the HER2/HER4 signaling is activated in myocytes due to cardiac stress by chemotherapy. Then, the inhibition of HER2 by trastuzumab impairs the response to myocardial damage, which may provoke the development of cardiac dysfunction (Di Cosimo 2011). The pooled incidence of trastuzumab-related cardiotoxicity, obtained from 15 randomized controlled trials and case control studies, was reported to be 10 %, whereas the pooled incidence of cardiotoxicity in studies with a non-trastuzumab comparator arm was 2 % (Panjrath and Jain 2007).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


