Atrioventricular Septal Defects
Frank Cetta
Dongngan Truong
L. Luann Minich
Joseph J. Maleszewski
Patrick W. O’Leary
Joseph A. Dearani
Harold M. Burkhart
Introduction and Nomenclature
Atrioventricular septal defects (AVSDs) are a group of anomalies that share a defect of the atrioventricular septum and abnormalities of the atrioventricular (AV) valves. The terms “atrioventricular canal defects” or “endocardial cushion defects” also describe these lesions but, for the purposes of this chapter, the term atrioventricular septal defect will be used. These lesions are divided into partial and complete forms. In partial AVSD, a primum atrial septal defect (ASD) is present and there are two distinct, but focally contiguous, right and left AV valve orifices. The left AV valve invariably is cleft. The complete form also includes a primum ASD, but it is contiguous with a large inlet ventricular septal defect (VSD) and the common AV valve has a single orifice. Similarly, the clinical manifestations and management of these patients vary.
Several classification schemes have been used to describe AVSDs. Transitional AVSD is a subtype of partial AVSD. This term is used when a partial AVSD also has a small/restrictive inlet VSD that is partially occluded by dense chordal attachments to the ventricular septum. Intermediate AVSD is a subtype of complete AVSD that has distinct right and left AV valve orifices and a large inlet VSD. These separate orifices are referred to as “right” and “left AV valves” rather than tricuspid and mitral. This also holds when describing the valves after repair of AVSD. The VSD in intermediate AVSD is large similar to other forms of complete AVSD. Unfortunately, the terms “transitional” and “intermediate” have been confused and are sometimes used synonymously in the literature. Because of the conflicting and confusing terminology of these subtypes, the clinician, echocardiographer, and surgeon should communicate by simply describing AV valve morphology, cardiac chamber sizes, and magnitude of shunting observed. It is useful to note, that complete and intermediate AVSD have the physiology and clinical features of a combined ASD and a large VSD. Partial and transitional AVSD have the clinical picture of a large ASD and no/restrictive VSD (Fig. 29.1).
Anatomic features shared by all forms of AVSD are summarized in Table 29.1.
Anatomic features shared by all forms of AVSD are summarized in Table 29.1.
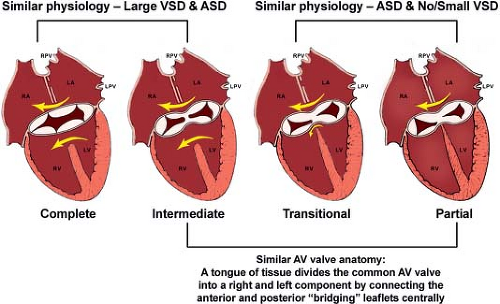 Figure 29.1 Summary of AVSD. Anatomic and physiologic similarities between the different forms of atrioventricular septal defect (AVSD) are illustrated. Complete AVSDs have one orifice with large interatrial and interventricular communications. Intermediate defects (one annulus, two orifices) are a subtype of complete AVSD and have a large ventricular septal defect (VSD). Complete AVSDs have physiology of VSDs and atrial septal defects (ASDs). In contrast, partial AVSDs have physiology of ASDs. Transitional defects are a form of partial AVSD in which a small inlet VSD is present or the ventricular level shunt has been obliterated by chordal tissue. Partial AVSDs and the intermediate form of complete AVSD share a similar anatomic feature: A tongue of tissue divides the common atrioventricular valve into distinct right and left orifices. Not pictured is a more rare form of AVSD that has a large inlet VSD and no primum ASD (see Figure 29.3). LA, left atrium; LPV, left pulmonary vein; LV, left ventricle; RA, right atrium; RPV, right pulmonary vein; RV, right ventricle. (Used with permission of Mayo Clinic Foundation.) |
TABLE 29.1 Anatomic Features Shared by All Forms of AVSD | ||||||
|---|---|---|---|---|---|---|
|
Surgical repair of AVSDs has been one of the great successes of the last several decades of congenital cardiac surgery. Recently, the Pediatric Heart Network (PHN) reported AVSD outcome data from seven North American centers demonstrating an overall operative mortality of 3% (1,2). Long-term survival has been excellent. Cumulative 20-year survival of 95% has been reported (3), with 30-year survival of 94% specific to patients with partial AVSD (4). This success has been tempered by the relatively large number of patients (approximately 25%) who await reoperation, most commonly because of progressive left AV valve regurgitation or left ventricular outflow tract (LVOT) obstruction. Unfortunately, the prevalence of postoperative left AV valve regurgitation has remained the most common sequel of repair for decades. The PHN study found that 26% of patients had more than moderate left AV regurgitation 6 months after “repair” (1,2).
Demographics
AVSDs account for 4% to 5% of congenital heart defects with an estimated occurrence of 0.19 in 1,000 live births (5,6). In a large fetal echocardiography experience, AVSD was the most common anomaly detected, constituting 18% of abnormal fetal hearts (7). In utero diagnosis of an AVSD is made on routine fetal four-chamber imaging. Gender distribution is approximately equal or may show a slight female preponderance (5).
Approximately 40% to 45% of children with Down syndrome have congenital heart disease, and among these, 45% have an AVSD. Most patients with Down syndrome, who have congenital heart disease, have the complete form of AVSD (>75%) (8). Conversely, 50% of patients with AVSD have Down syndrome. Race and ethnicity may factor into prevalence of AVSD in patients with Down syndrome. Patients of Black African ancestry with Down syndrome have AVSD more commonly than do whites. Hispanics with Down syndrome appear to have AVSD less commonly than whites (9). Familial occurrence of AVSD is rare. Complete AVSD also occurs in patients with heterotaxy (more common with asplenia than polysplenia) and Ellis–van Creveld syndromes. Common atrium has been associated with Ellis–van Creveld and heterotaxy syndromes (10,11,12). In utero diagnosis of AVSD is common and there is a high rate of associated extracardiac manifestations. The group from Stanford recently reported that overall mortality rate for fetuses with AVSD was 48% and the presence of extracardiac anomalies was an independent factor that predicted fetal or neonatal death (13).
Embryogenesis
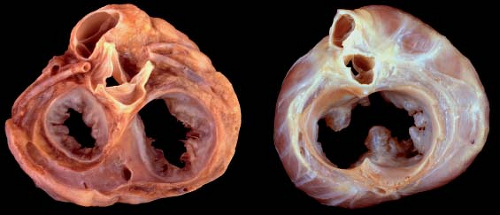
The etiology of AVSDs has traditionally been considered due to faulty development of the AV endocardial cushions. However, recent studies have illustrated additional pathways involving the “dorsal mesenchymal protrusion” that may act alone or in concert with development of the endocardial cushions to create AVSDs (14). In partial AVSDs, incomplete fusion of the superior and inferior endocardial cushions results in a cleft in the midportion of the left AV valve anterior leaflet often associated with regurgitation. In contrast, complete AVSD is associated with lack of fusion between the superior and inferior cushions and, consequently, with the formation of separate anterior and posterior bridging leaflets along the subjacent ventricular septum.
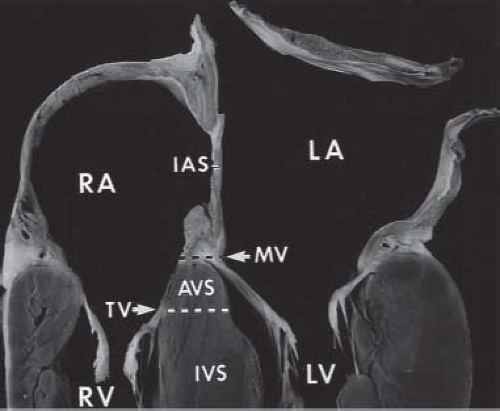
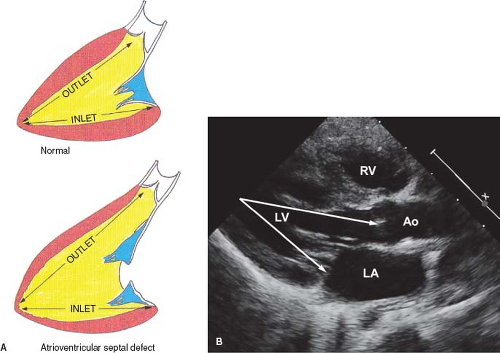
Failure of the endocardial cushions to fuse creates a defect in the AV septum. The primum atrial septal component of this defect is variable in size and can be quite large. This results in downward displacement of the anterior left AV valve leaflet to the level of the septal right AV valve leaflet (15). In AVSDs, the AV valves have the same septal insertion level in contrast to the leaflet arrangement in the normal heart (Fig. 29.2). The distance from the cardiac crux to the left ventricular apex is foreshortened, and the distance
from the apex to the aortic valve is increased. This is in contrast to the normal heart, in which the two distances are roughly equal (Fig. 29.3A, B). In AVSDs, the disproportion between the two distances causes anterior displacement of the LVOT resulting in elongation and narrowing of the LVOT producing the characteristic “gooseneck” deformity. After surgical repair of AVSD, progressive subaortic stenosis may develop (16).
from the apex to the aortic valve is increased. This is in contrast to the normal heart, in which the two distances are roughly equal (Fig. 29.3A, B). In AVSDs, the disproportion between the two distances causes anterior displacement of the LVOT resulting in elongation and narrowing of the LVOT producing the characteristic “gooseneck” deformity. After surgical repair of AVSD, progressive subaortic stenosis may develop (16).
Since the dextrodorsal conus cushion contributes to the development of the right AV valve and the outflow tracts lie adjacent to their respective inflow tracts, AVSDs may be associated with conotruncal anomalies, such as tetralogy of Fallot and double-outlet right ventricle. In addition, shift of the AV valve orifice may result in connection of the valve primarily to only one ventricle (usually the right ventricle), creating disproportionate or unbalanced ventricles.
Partial Atrioventricular Septal Defect
Pathology
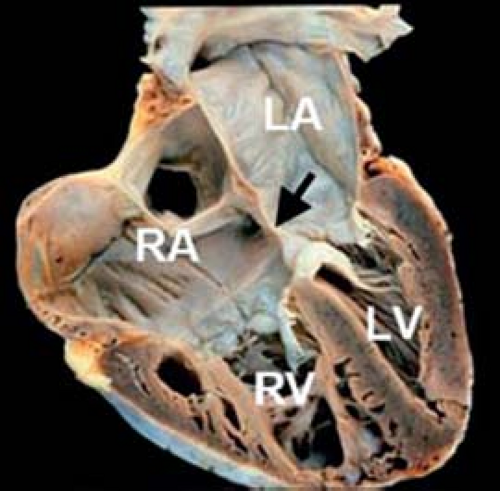
In partial AVSD, the right and left AV valve orifices are separated by a tongue of tissue. The most frequent form of partial AVSD consists of a primum ASD and a cleft left AV valve anterior leaflet (Figs. 29.4 and 29.5). Most primum ASDs are large and located anterior and
inferior to the fossa ovalis. The defect is bordered by a crescentic rim of atrial septal tissue posterosuperiorly and by AV valve continuity anteroinferiorly. These defects are not amenable to transcatheter device closure because of their proximity to the AV valves.
inferior to the fossa ovalis. The defect is bordered by a crescentic rim of atrial septal tissue posterosuperiorly and by AV valve continuity anteroinferiorly. These defects are not amenable to transcatheter device closure because of their proximity to the AV valves.
The right and left AV valves have the same septal insertion level because the left AV valve annulus is displaced toward the ventricular apex. As a result, the deficient AV septum is associated with an interatrial communication rather than an interventricular or right atrial to left ventricular communication. Nonetheless, the defect imparts a scooped-out appearance to the inlet ventricular septum, and the distance from the left AV valve annulus to the left ventricular apex is less than the distance from the aortic annulus to the apex (see Fig. 29.3).
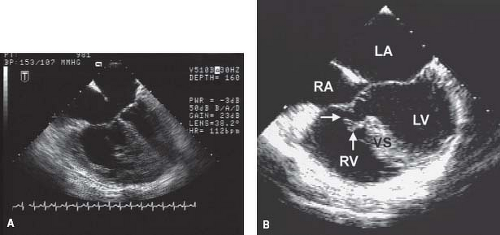
The cleft in the left AV valve anterior leaflet is directed toward the midportion of the ventricular septum, along the anteroinferior rim of the septal defect (Figs. 29.6 and 29.7). In contrast, isolated mitral valve clefts (not associated with AVSD) are directed toward the aortic valve annulus (17). The left AV valve orifice is triangular rather than the elliptical shape of a normal mitral valve and more resembles a mirror-image tricuspid valve orifice. The cleft left AV valve usually is regurgitant and, with time, becomes thickened and exhibits histologic alterations that resemble myxomatous mitral valve prolapse.
The most common associated anomalies with partial AVSD are secundum ASD, patent ductus arteriosus (PDA), and persistent left superior vena cava connecting to the coronary sinus (1). Less frequently, pulmonary stenosis, tricuspid stenosis or atresia, cor triatriatum, coarctation of the aorta, membranous VSD, pulmonary venous anomalies, and hypoplastic right or left ventricle have been reported (18,19,20).
Clinical Manifestations
Although patients with partial AVSD may be asymptomatic until adulthood, symptoms of excess pulmonary blood flow typically occur in childhood. Tachypnea and poor weight gain occur earlier and are more severe when the defect is associated with moderate or severe left AV valve regurgitation or with other hemodynamically significant cardiac anomalies. Compared with patients with secundum ASDs, patients with primum ASDs usually have earlier and more severe symptoms, including growth failure.
An uncomplicated primum ASD often is discovered in young children when echocardiography is performed to investigate a murmur. The murmur is caused by turbulent flow across the pulmonary valve (not flow through the ASD) and has typical systolic ejection qualities
best heard over the upper left sternal border with radiation to the lung fields. The second heart sound is widely split and fixed during respiration. A holosystolic murmur owing to left AV valve regurgitation may also be heard at the apex. A low-pitched mid-diastolic murmur heard at the left lower sternal border may be present if the shunt is large or if significant left AV valve regurgitation is present.
best heard over the upper left sternal border with radiation to the lung fields. The second heart sound is widely split and fixed during respiration. A holosystolic murmur owing to left AV valve regurgitation may also be heard at the apex. A low-pitched mid-diastolic murmur heard at the left lower sternal border may be present if the shunt is large or if significant left AV valve regurgitation is present.
Echocardiography
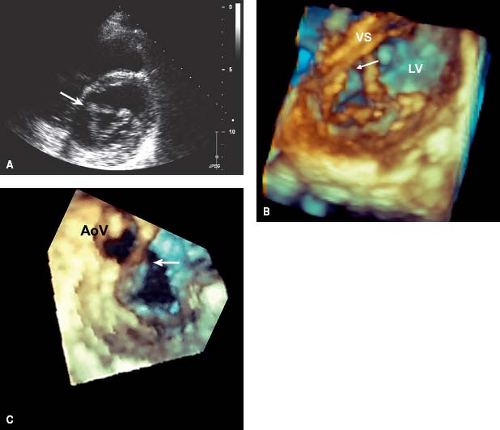
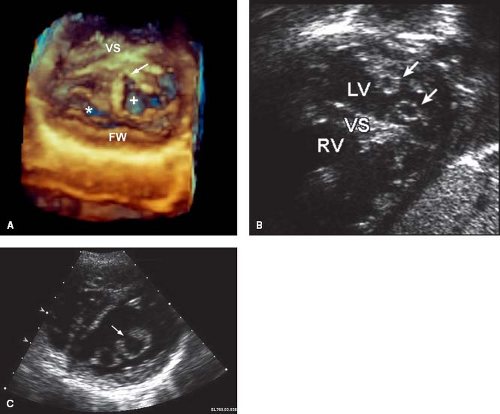
Two-dimensional (2-D) echocardiography is the primary imaging technique for diagnosing AVSDs (22,23,24). It is particularly useful for delineating the morphology of the AV valves. Transesophageal echocardiography (TEE) can provide incremental diagnostic information in larger patients or in patients with associated complex abnormalities. Three-dimensional (3-D) echocardiography can complement the 2-D evaluation, particularly in the setting of AV valve abnormalities, by providing greater detail of the leaflet morphology, cleft, commissures, and subvalvular apparatus (25,26). Three-dimensional echocardiography has the unique advantage of allowing imaging of the left AV valve in orientations that are not possible using 2-D techniques, including a view of the valve from the left atrium—a “surgical view” (26).
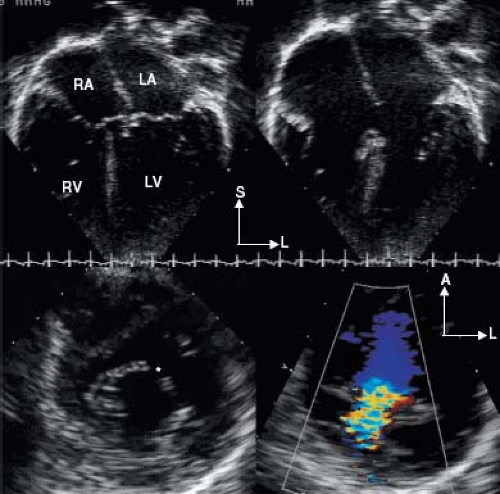
The internal cardiac crux is the most consistent echocardiographic imaging landmark (21). The apical four-chamber imaging
plane clearly allows visualization of the internal crux where the primum ASD is seen as an absence of the lower atrial septum. The size of the primum ASD is made reliably from this imaging position (Fig. 29.8) or from the subcostal four-chamber (frontal) projection. Accurate visualization of the cardiac crux also permits assessment of the AV valves. Several 2-D echocardiographic features are shared by all forms of AVSD: deficiency of a portion of the inlet ventricular septum, inferior displacement of the AV valves, and attachment of a portion of the left AV valve to the septum. The AV valves are displaced toward the ventricles, with the septal portions inserting at the same level onto the crest of the ventricular septum. Therefore, the two separate AV valve orifices are equidistant from the cardiac apex.
plane clearly allows visualization of the internal crux where the primum ASD is seen as an absence of the lower atrial septum. The size of the primum ASD is made reliably from this imaging position (Fig. 29.8) or from the subcostal four-chamber (frontal) projection. Accurate visualization of the cardiac crux also permits assessment of the AV valves. Several 2-D echocardiographic features are shared by all forms of AVSD: deficiency of a portion of the inlet ventricular septum, inferior displacement of the AV valves, and attachment of a portion of the left AV valve to the septum. The AV valves are displaced toward the ventricles, with the septal portions inserting at the same level onto the crest of the ventricular septum. Therefore, the two separate AV valve orifices are equidistant from the cardiac apex.
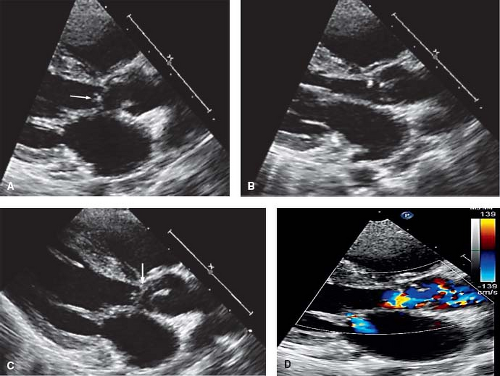
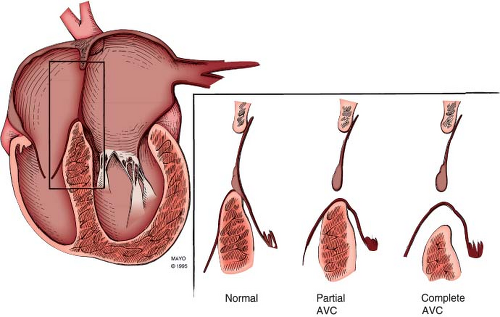 Figure 29.8 AVSD and the internal cardiac crux. The internal cardiac crux is best visualized in the apical four-chamber plane. In the normal heart, the anterior leaflet of the mitral valve insertion is more superior than the tricuspid septal leaflet. In all forms of AVSD, both right and left atrioventricular valve components insert at the same level, usually on the crest of the inflow ventricular septum, except in the rare cases of large inlet VSD without a primum ASD, when they are attached to the atrial septum (see Fig. 29.23). A cleft in the left AV valve occurs in conjunction with the downward displacement of the anterior leaflet. In partial AVSD, there is a defect in the lower fatty portion of the atrial septum (i.e., within the atrial ventricular septum). In complete AVSD, there is a defect beneath the atrioventricular valves in the inflow ventricular septum. In general, these easily recognized anatomic features distinguish normal from partial and complete AVSD. AVC, atrioventricular canal. |
In the transitional form of partial AVSD, there is aneurysmal replacement of a portion of the inlet ventricular septum (Fig. 29.9) (23). Small shunts may occur through this so-called “tricuspid pouch.” However, these dense chordal attachments eventually obstruct flow. Spectral and color flow Doppler hemodynamic assessments are useful to determine the severity of AV valve stenosis or insufficiency and to quantitate right ventricular systolic pressure. Doppler echocardiography is not useful for evaluating the degree of left AV valve stenosis in the setting of a primum ASD because the ASD decompresses the left atrium.
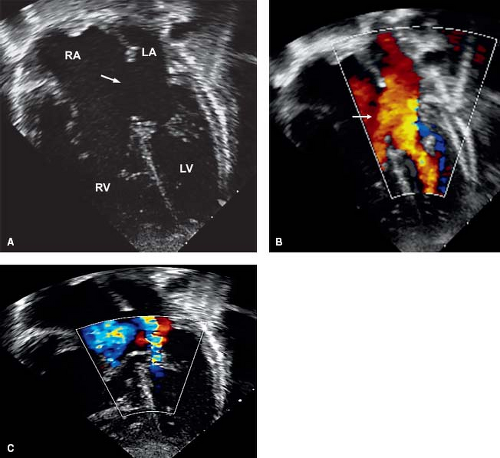
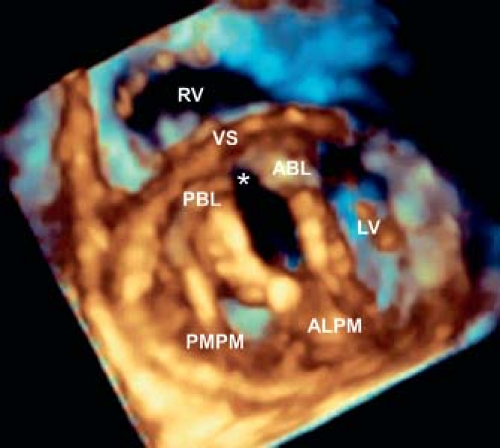
Left AV valve abnormalities occur both in the partial and complete forms of AVSD (Figs. 29.10 to 29.12; Videos 29.1 and 29.2). The most common abnormality, a cleft, is best visualized from the parasternal and subcostal short-axis imaging planes. Careful 2-D and color Doppler assessment of the left AV valve cleft needs to be performed since multiple jets of regurgitation may be present. Three-dimensional transthoracic imaging visualizes the cleft from both the atrial and ventricular aspects (Fig. 29.10B, C). Since 2-D imaging can overestimate the number of regurgitant jets in preoperative and postoperative patients with AVSD (25), 3-D color Doppler visualization of the left AV valve from the atrial side can be used to provide more accurate quantification of the regurgitant jets. Left AV valve regurgitation that is directed into the right atrium is usually remedied with successful repair of the cleft and closure of the primum ASD.
In contrast, left AV valve regurgitation into the left atrium may not be completely eliminated with cleft repair and may signify other intrinsic abnormalities with the valve. An important factor that predicts the amount of residual postoperative left AV valve regurgitation is the degree of preoperative regurgitation. Rarely other abnormalities of the left AV valve occur such as a parachute or a double-orifice valve.
Double-orifice left AV valve occurs in 3% to 5% of AVSDs (27) and is more common when two distinct right and left AV valve orifices are present. This valve anomaly is created when a tongue of tissue divides the left AV valve into two orifices. The combined effective valve area of a double-orifice valve always is less than the valve area of a single-orifice left AV valve, this predisposes the valve to
postoperative stenosis. Standard subcostal and parasternal short-axis views usually demonstrate the double-orifice valve characteristics (see Fig. 29.11B, C). Three-dimensional echocardiography clarifies the anatomy (see Fig. 29.11A), particularly in the setting of a common AV valve, when this diagnosis may be challenging. Double-orifice left AV valve rarely occurs in hearts that do not have AVSD. In contrast to AVSD, in the otherwise normal heart, double-orifice mitral valves have complete duplication of the valve structure.
postoperative stenosis. Standard subcostal and parasternal short-axis views usually demonstrate the double-orifice valve characteristics (see Fig. 29.11B, C). Three-dimensional echocardiography clarifies the anatomy (see Fig. 29.11A), particularly in the setting of a common AV valve, when this diagnosis may be challenging. Double-orifice left AV valve rarely occurs in hearts that do not have AVSD. In contrast to AVSD, in the otherwise normal heart, double-orifice mitral valves have complete duplication of the valve structure.
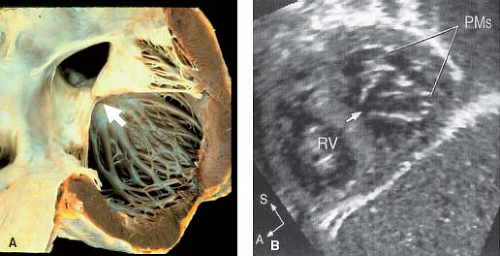
The supporting, subvalvular apparatus of the left AV valve may be an underappreciated contributor to both preoperative and postoperative left AV valve regurgitation in partial and complete AVSDs. In 2010, Ando and Takahashi (28) assessed anatomic factors associated with more difficult VSD patch placement or cleft approximation in 138 patients with complete AVSD and identified four risk factors for at least moderate left AV valve regurgitation: (1) papillary muscle abnormalities (see Fig. 29.12), most commonly imbalance of the papillary muscles; (2) dense chordal attachments of the anterior bridging leaflet obscuring the right side of the crest of the ventricular septum; (3) double-orifice left AV valve; and (4) severe length discrepancies of the cleft. Using 3-D echocardiographic quantitative measures to assess risk factors for left AV valve regurgitation, Takahashi et al. (29) found that a laterally (i.e., basally displaced) anterolateral papillary muscle was associated with at least moderate or greater left AV valve regurgitation. Colen et al. (30) extended the preoperative understanding of the left AV valve by using 3-D echocardiography of the left AV valve to describe a constellation of abnormalities in patients with partial and complete AVSD including dominant anterolateral papillary muscle with short, thickened, or absent chords, eccentrically positioned cleft, and blunted or deformed anterior bridging mural commissure not appreciated on 2-D assessment. These details allow the surgeon to consider alternatives to the standard surgical approach before instituting cardiopulmonary bypass (CPB).
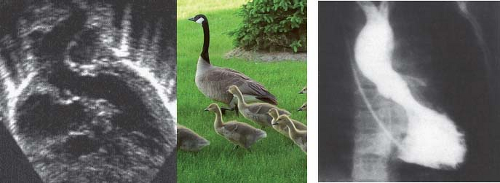
In the normal heart, the aortic valve is wedged between the mitral and tricuspid annuli. In the heart with AVSD, the aortic valve is displaced or “sprung” anteriorly (Fig. 29.13). This anterior displacement creates an elongated, so-called “gooseneck” deformity of the LVOT (Fig. 29.14) that predisposes the patient to
progressive subaortic obstruction. LVOT obstruction may occur in all subtypes of AVSD, but is more frequent when two distinct AV valve orifices are present rather than when there is a common orifice. This may result from the superior bridging leaflet attaching to the crest of the ventricular septum, creating two orifices and even further elongating and narrowing the LVOT. Discrete subaortic fibromuscular ridges, septal hypertrophy, abnormal left AV valve chordal attachments, and abnormally oriented papillary muscles can further exacerbate the subaortic narrowing. LVOT obstruction may be subtle and therefore not appreciated during the preoperative echocardiographic assessment. LVOT obstruction may also develop de novo after initial repair of AVSD (Fig. 29.15) (31), even after primary repair of partial AVSD in adulthood. Mayo Clinic reported the case of a 58-year-old woman who required reoperation for LVOT obstruction after primary repair of partial AVSD at age 45 years (32). LVOT obstruction often is progressive.
progressive subaortic obstruction. LVOT obstruction may occur in all subtypes of AVSD, but is more frequent when two distinct AV valve orifices are present rather than when there is a common orifice. This may result from the superior bridging leaflet attaching to the crest of the ventricular septum, creating two orifices and even further elongating and narrowing the LVOT. Discrete subaortic fibromuscular ridges, septal hypertrophy, abnormal left AV valve chordal attachments, and abnormally oriented papillary muscles can further exacerbate the subaortic narrowing. LVOT obstruction may be subtle and therefore not appreciated during the preoperative echocardiographic assessment. LVOT obstruction may also develop de novo after initial repair of AVSD (Fig. 29.15) (31), even after primary repair of partial AVSD in adulthood. Mayo Clinic reported the case of a 58-year-old woman who required reoperation for LVOT obstruction after primary repair of partial AVSD at age 45 years (32). LVOT obstruction often is progressive.
Detailed and comprehensive echocardiographic assessment is required to evaluate associated lesions and determine their significance. Tetralogy of Fallot, double-outlet right ventricle, and pulmonary valve atresia are associated with all forms of AVSDs but are less frequent with partial AVSD. In contrast, AV valve abnormalities and left ventricular hypoplasia are more frequent in two-orifice AV connections. Coarctation of the aorta occurs with equal frequency in partial and complete AVSD.
Radiography
Chest radiography demonstrates cardiomegaly and prominent pulmonary vascular markings. Because the jet of left AV valve regurgitation often is directed into the right atrium, right atrial enlargement rather than left atrial enlargement may be apparent.
Electrocardiography and Electrophysiology
The position of the AVSD dictates the position of the AV conduction tissues. Accordingly, the AV node is displaced posteriorly, near the orifice of the coronary sinus, and the His bundle is displaced inferiorly, along the inferior rim of the septal defect. The displacement of the AV conduction tissues, in conjunction with loss of ventricular septal myocardium, results in left axis deviation in the frontal plane QRS.
Sinus rhythm is present in most patients with a primum ASD. Prolongation of the P-R interval, in relation to patient age and heart rate, is seen in approximately 25% of patients. It is due primarily to increased conduction time from the high right atrium to the low septal right atrium (33,34). P-wave changes indicating right atrial, left atrial, or biatrial enlargement are seen in approximately half of patients. The mean QRS axis in the frontal plane ranges from −30 to −120 degrees, with most axes between −30 and −90 degrees. Anatomic and electrophysiologic studies show that this abnormal vectorcardiographic pattern is associated with a specific anomaly of the conduction system (34). Right ventricular volume overload results in right ventricular hypertrophy and some variation of the rsR′ or RSR′ pattern in the right precordial leads in 80% of patients; 10% of patients have a qR pattern. Patients with significant left AV valve regurgitation may have evidence of left ventricular hypertrophy.
Except for prolonged intra-atrial conduction time, other intracardiac electrophysiologic measurements usually are normal, including sinus node function, AV node function, His–Purkinje conduction time, and refractory periods (33).
Cardiac Catheterization and Angiography
Cardiac catheterization and angiography are rarely required for diagnosis or management of patients with a partial AVSD. Current echocardiographic techniques (including 3-D reconstruction) accurately define the anatomy and physiology of this lesion. In an older patient, cardiac catheterization may have a role in assessing the degree of pulmonary vascular obstructive disease or coronary artery disease.
A large left-to-right shunt can be demonstrated at atrial level by a higher oxygen saturation sampled from the right atrium compared with the blood in the inferior and superior vena cavae. Because of the anatomic position of the ASD, blood samples taken from the inflow portion of the right ventricle may have elevated oxygen saturation. The calculated left-to-right shunt often exceeds 50%. In most patients, right ventricular pressure is <60% of systemic pressure. A significant increase in calculated pulmonary vascular resistance is unusual in infants. Left ventricular angiography demonstrates the “gooseneck deformity” of the LVOT (see Fig. 29.14).
Special Forms of Partial Atrioventricular Septal Defect
Malaligned Atrial Septum or Double-Outlet Right Atrium
Deviation of the atrial septum to the left of the AV junction rarely has been reported (35,36,37). When this occurs, both the right and left AV valves are visualized from the right atrium and connected to both ventricles through a large primum ASD. If deviation of the atrial septum to the left is extreme, the pulmonary veins may be isolated and obstructed, simulating cor triatriatum. The term “double-outlet” right atrium has been applied to this lesion (36); it is a form of AVSD.
Common Atrium
Common atrium is characterized by near absence of the atrial septum. In the presence of two ventricles, it always is associated with an AVSD (38). Cases of common atrium typically are syndromic, most commonly having heterotaxy or Ellis–van Creveld (10,11,12). The pathologic spectrum of this lesion ranges from patients with coexistent primum and secundum ASDs to others who lack the entire septum except for a small muscular cord. Patients with syndromes and common atrium frequently have concomitant complex congenital heart disease. In these cases, transposition of the great arteries, double-outlet right ventricle, univentricular AV connection, and anomalous pulmonary venous connections are encountered. Patients with common atrium have splenic abnormalities and anomalies of cardiac and abdominal sidedness (situs).
Clinical Manifestations
Most patients with common atrium present in infancy with symptoms of excess pulmonary blood flow: fatigue, tachypnea, and failure to thrive. However, if increased pulmonary vascular resistance develops, the left-to-right shunt decreases and somatic growth improves. In general, these patients are symptomatic earlier in life than patients with an isolated primum ASD. The radiographic and electrocardiographic characteristics of patients with common atrium are indistinguishable from those with other forms of AVSD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree