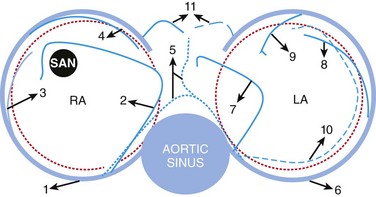
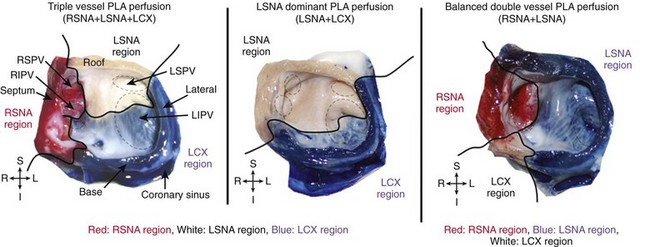
44 Atrial fibrillation (AF) is the most common arrhythmia in adults and affects more than 4 million Americans.1,2 This arrhythmia is associated with such adverse consequences as decreased quality of life, decompensated cardiovascular status, and stroke.3,4 Despite the availability of antiarrhythmic drugs and interventional therapies, AF still represents a therapeutic challenge, and costs related to AF management steadily increase.5–9 AF may be caused by any cardiac condition; however, a predominance of heart failure and coronary artery disease (CAD) has been noted.1,10–14 Regarding CAD-associated AF, clinical and experimental studies show that both acute and chronic CAD lead to onset and perpetuation of AF.11,15–18 For instance, AF is a well-known complication of acute myocardial infarction,18 and CAD is a significant risk factor for AF.8 Although AF after ventricular myocardial infarction might also be triggered by an increase in intra-atrial pressure in the context of acute ventricular dysfunction,19,20 various works have shown that isolated atrial infarction is common, representing about 20% of autopsy-proven atrial infarctions.21,22 Recent years have seen a growth of interest in further understanding the mechanisms by which atrial ischemia, acute or prolonged, may favor onset and perpetuation of AF. In this chapter, we review the anatomical and physiological background of atrial coronary perfusion and present the most recent insights demonstrating a causative link between atrial ischemia and AF. Available literature on the detailed anatomy of atrial coronaries is scarce, and unstandardized nomenclature makes any attempt at gaining understanding challenging. In general the sinus nodal artery (SNA), the atrioventricular nodal artery (AVNA), and several other arterial branches in the atria have been described. Although these play a significant role in the pathophysiology of atrial fibrillation and other tachyarrhythmias, they have no unanimous characterization in the cardiology literature, most likely owing to the variability of their origin, course, and termination. Figure 44-1 shows schematically the atrial coronary branches and anastomoses that have been reported in the literature. Figure 44-1 Atrial arterial branches. 1, Right coronary artery; 2, right anterior atrial branch (main atrial branch in this figure); 3, right intermediate branch; 4, right posterior branch; 5, Kugel arterial anastomosis; 6, left circumflex artery; 7, left anterior atrial branch; 8, left intermediate atrial branch; 9, left posterior branch; 10, left atrial circumflex; 11, atrioventricular nodal artery. LA, Left atrium; RA, right atrium; SAN, sinoatrial node. (Adapted from Boppana S et al: Atrial coronary arteries: Anatomy and atrial perfusion territories J Atrial Fibrillation September 2011.) Arteries supplying the right atrium (RA) are among the earliest branches of the right coronary artery (RCA) after the conus artery, and they originate along the right atrioventricular (AV) groove (see Figure 44-1). They are usually classified into the right anterior, intermediate/marginal, and posterior atrial branches (Spalteholz classification).23 Angiographically, in a right anterior oblique (RAO) projection, the right anterior atrial branch is slender, arises at a variable distance from the conus artery, and courses to the left and superior aspect of the atrium.12 It gives off branches to both atria and the interatrial septum and terminates by encircling the lower portion of the superior vena cava (SVC). When it is the main atrial branch, it supplies the sinoatrial node (SAN). The right intermediate atrial branch, also called the marginal branch, arises from the acute right margin of the heart, ascends over the anterolateral surface of the RA, and supplies surrounding atrial tissues (see Figure 44-1). In 13.3% of cases, it is the main atrial branch that supplies the sinus node.13,14 The origin of this branch is marked by the presence of a small fatty excrescence or a cardiac vein adjacent to the artery that drains into the RA wall.15 Angiographically, the right intermediate atrial branch is not easy to see because of its small size and variability. For the same reason, the right posterior atrial branch is difficult to locate. When present, the right posterior branch arises on the posterior aspect of the RA and supplies the right posterior atrial wall and the left atrial (LA) posterior surface. Notably Busquet et al proposed another classification scheme in which right atrial branches are classified on the basis of cross-sectional diameter into two rather than three groups—the major and accessory branches.15 Major branches are usually found in groups occupying anterior, lateral, and posterior positions relative to the tricuspid orifice, consistent with the Spalteholz classification. Accessory branches arise from the RCA in the AV groove and have an average diameter of less than 0.5 mm and a length not exceeding 10 mm. They are covered by fatty tissue in the AV groove and supply solely the lower atrial wall and the adjacent right ventricle. Arteries supplying the LA are among the earliest branches of the left coronary artery (LCA), usually from the left circumflex (LCX), and originate along the AV groove. Similar to right atrial circulation, left atrial branches are usually classified into the left anterior, intermediate/marginal, and posterior atrial branches. The left anterior atrial branch arises on the anterior aspect of the LA (see Figure 44-1). When it is the main atrial branch, it courses upward along the LA via the anterior interatrial groove to reach the SAN. In RAO projection, it is an early branch that ascends leftward and upward.12 The remaining left atrial branches are variable and supply adjacent atrial tissues along their course over the LA. Another variant is the left atrial circumflex artery (LACX), which may branch early from either the LCX or its main atrial branch (Figure 44-1).12,13 Early in its course, the LACX ascends slightly through the LA and travels along its lower margin parallel to the left AV groove. It extends around the left heart margin and terminates in the posterior wall of the LA. Sometimes, the LACX extends farther, crossing the crux of the heart along the right AV groove to give off right posterior atrial branches. Rarely, the LACX may even supply the SAN as the main atrial branch itself, which, in a study of 118 patients, occurred 11% of the time.13 Several anastomoses between atrial coronary arterial systems have been described. Generally, these anastomoses exist as small intra-atrial or atrioventricular branches, or as a single vessel known as Kugel’s artery—a major transatrial pathway that bridges the right and left coronary systems (see Figure 44-1). As the largest atrial artery, the SNA also serves as a major anastomotic channel, connecting to right and left coronary systems throughout its course. It forms a vascular loop around the base of the SVC, where it sends tributaries to small branches of the left and right intermediate arteries.15,20 When present, the posterior termination of the LACX can also anastomose with the distal RCA or AVNA.16 Many authors who accepted the importance of an anastomotic network between the left and right coronary systems rejected the notion that the anastomosis was formed by a single major vessel.21,22 The debate was further confounded by their usage of the name “Kugel’s artery” for their descriptions of different anastomotic networks. Reconciling these diverging viewpoints, T.N. James described only two variations of Kugel’s vessel.20 He reported an arterial connection between either the proximal LCX or RCA with vessels in the crux. Later, these were referred to as the left and right Kugel’s artery, respectively, and are commonly accepted today as such.23 Angiographically, the left anterior oblique projection best identifies this anastomotic network, which appears as a flag-like rectangular termination.24 Although the reports described here have focused on a description of the origin, course, and termination of atrial coronary arteries, the precise extent of atrial perfusion territories has received little attention. Specifically, a delineation of atrial perfusion territories at the posterior wall of the LA (PLA) and the pulmonary veins (PVs), which are crucial regions for AF maintenance, has received little attention. Our group conducted an investigation in isolated ovine hearts to address this question.24 In good accordance with human descriptions,33 we and others first established that in sheep hearts, three atrial coronary branches—left SNA (LSNA), right SNA (RSNA), and atrial branches of the LCX—contribute to the perfusion of the PLA including PV regions.24,25 We then delineated atrial coronary perfusion territories by selective perfusion of Congo red into the RSNA, and Evan’s blue into the LCX (Figure 44-2, left and middle panels) or the LSNA (right panel). Both atria including the PLA and the PVs were then dissected for acquisition of photographic snapshots. As shown in Figure 44-2, each atrial branch (RSNA, LSNA, and LCX branches) irrigated a well-defined perfusion territory at the PLA and within the pulmonary veins. However, the contribution of individual arteries to the perfusion of the PLA-PV myocardium varied between specimens, and three equally important anatomical variants were most often observed: triple-vessel PLA perfusion (Figure 44-2 left; 29.5%), double-vessel PLA perfusion (right; 23.5%), and one-vessel PLA perfusion (middle; 29.5%). These data suggest that the complexity of atrial vessel origin and course may translate into intricate perfusion areas in regions well known to harbor electrical sources of AF initiation and maintenance. This also indicates that advanced coronary artery disease may associate with atrial infarctions involving variable regions of the atria. Figure 44-2 Atrial coronary perfusion territories in sheep: Main anatomical variants. Left, Triple-vessel PLA perfusion; middle, LSNA-dominant PLA perfusion; right, balanced double-vessel PLA perfusion. Black solid line delineates regional perfusion territories. Left panel, Selective perfusion of Congo red into the RSNA, and Evans blue into the LCX; middle panel, selective perfusion of Congo red into the RSNA (no left atrial staining) or the LSNA (right panel). Right panel, Selective perfusion of Congo red into the RSNA and Evans blue into the LSNA. LIPV, left inferior pulmonary vein; LSNA, left sinus node artery; LSPV, left superior pulmonary vein; PLA, posterior wall of the left atrium; RIPV, right inferior pulmonary vein; RSNA, right sinus node artery; RSPV, right superior pulmonary vein. (Adapted from Yamazaki M et al: Left atrial coronary perfusion territories in isolated sheep hearts: implications for atrial fibrillation maintenance. Heart Rhythm 7:1501–1508, 2010.) The thickness of the atrial wall has been reported to range from 0.5 to 4 mm26,27; thus it is conceivable that the blood present within the atrial cavities succeeds in superfusing the thinnest regions. However, available literature indicates that oxygen may diffuse to only a few layers of myocytes. A histologic study of capillary density in the left atrial free wall and the left atrial appendage of patients in chronic AF showed that the maximal oxygen diffusion distance was estimated at 15 to 20 µM.28 Others have suggested that up to 500 µM of myocardial wall may be adequately superfused.29 Nonetheless, a large proportion of the atrial wall remains dependent on an efficient coronary perfusion, and a perfusion impediment will translate into an infarcted region spanning most of the atrial wall. Atrial infarction has been extensively described in the literature, although it remains an elusive and difficult diagnosis to make clinically.30,31 Although atrial infarction is fairly common in the setting of acute myocardial infarction (AMI),32–34 the rate of 17% reported by Cushing et al in 1942 in the largest study to date may be the best estimate of its occurrence.21 However, these numbers, derived from postmortem studies, suggest that the actual clinical incidence rate might be higher.35 In fact, it was suggested that the pathophysiological role of atrial ischemia-infarction in AF onset is greatly underestimated.36,37
Atrial Ischemia and Fibrillation
Atrial Perfusion: Anatomy and Physiology
Atrial Coronary Arteries and Branches
Right Atrial Branches
Left Atrial Branches
Right and Left Atrial Anastomoses and the Controversial Kugel’s Artery
Atrial Perfusion Territories
Role of Atrial Myocardium Superfusion
Atrial Fibrillation and Myocardial Ischemia-Infarction
Atrial Infarction, Decreased Atrial Coronary Flow
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Atrial Ischemia and Fibrillation
