CHAPTER
9
Atrial Fibrillation
UNDERSTANDING AND EVALUATING ATRIAL FIBRILLATION (AF)
Anatomy and Pathophysiology (Mechanism)
○Structural (fibrosis, hypertrophy, dilation, etc.) and/or electrophysiologic abnormalities (ion current or connexin changes affecting action potential duration [APD] and/or conduction) promote abnormal impulse formation and/or propagation.
○After the onset of AF, there are immediate (within minutes) and early (hours to days) alterations in the atrial electrophysiological properties (shortening of the atrial APD and ERP, abnormal action potential rate adaptation), followed by later changes in atrial structure (e.g., fibrosis, dilation) and mechanical function.
▪ These changes are largely because of the down-regulation of the L-type inward calcium current, impaired intracellular calcium release, up-regulation of inward rectifier potassium current, and alterations of myofibrillary energetics.
Hypotheses Regarding the Initiation and Perpetuation of AF
○Initiation (triggers):
▪ Ectopic foci initiate rapid repetitive discharges due to triggered activity (delayed > early afterdepolarization) or enhanced automaticity.
▪ The predominant triggers are located in the pulmonary veins (PVs).
• The PVs contain sleeves of left atrial (LA) myocardium:
▫ These sleeves extend ~1.5–2.5 cm beyond the LA–PV junction.
▫ These sleeves are thickest in the carina and venoatrial junction (mean 1.1 mm).
▫These sleeves are longer in the superior PVs with the left superior pulmonary vein [LSPV] sleeve being longer than the right superior pulmonary vein [RSPV] sleeve.
• The sleeves have shorter effective and functional refractory periods compared to LA, with critical zones of conduction delay and non-uniform anisotropy related to abrupt changes in fiber orientation.
○Perpetuation (substrate)
▪ Multiple-wavelet hypothesis: Postulates that AF results from the continuous annihilation and regeneration of multiple independent coexisting wavelets propagating randomly throughout the atria. It suggests that AF could be indefinitely perpetuated as long as the atrium had a sufficient electrical mass to prevent the simultaneous termination of all of the reentrant activity.
▪ Localized-source hypothesis: Postulates that AF is perpetuated by discrete, organized and rapid reentrant circuits (e.g., spiral wave reentrant circuits or “rotors”), or focal impulses that disorganize into fibrillatory waves at their periphery (e.g., drivers close to cardiac ganglion plexi with central organized activation and surrounding variable propagation and fractionation).
Classification
○ First detected episode
○ Paroxysmal: An AF that terminates spontaneously within 7 days of onset is defined as paroxysmal. According to some definitions, AF terminated by electrical or pharmacological cardioversion within 7 days is also considered paroxysmal.
○ Persistent: Episodes that last longer than 7 days
○ Longstanding persistent: Continuous AF of longer than 12 months duration
○ Permanent: Acceptance of AF (decision to cease further attempts of rhythm control)
○ Lone: AF in patients <60 years old with no structural heart disease
○ Non-valvular AF: AF in the absence of rheumatic mitral stenosis or a mechanical heart valve
Epidemiology and Clinical Features
○AF is the most common sustained arrhythmia seen in clinical practice.
▪ Prevalence: 1%–2% of the population
• Increases with age: <1.0% at 50 years, 1%–4% at 65 years, and 6%–15% at 80 years
• Less common in women: Male sex is associated with a 1.5× increased risk.
• Lifetime-risk of developing AF for individuals 40–55 years of age is estimated to be 22%–26%.
▪ AF accounts for 1.0%–2.7% of total annual healthcare expenditures.
○AF is associated with:
▪ Reductions in quality of life, functional status, and cardiac performance
▪ Reduced overall survival (RR of death with AF is 1.4–3.0)
▪ Increased risk of cardiac thromboembolism
• Valvular AF (rheumatic mitral stenosis [MS] or mechanical heart valve): Up to 17× increased risk of stroke (vs. sinus rhythm)
• Non-valvular AF: 3.5× increased risk of stroke (vs. sinus rhythm)
• Note: AF-related strokes are often recurrent and relatively more severe, causing significantly greater long-term disability, and mortality (25:1 hemispheric to transient ischemic attack [TIA] ratio vs. 2:1 for carotid sources).
▪ Increased risk of cognitive dysfunction
• There is a 1.7- to 3.3-fold increased risk of cognitive impairment (vs. sinus rhythm).
• There is a 2.3-fold increased risk of dementia (vs. sinus rhythm).
Table 9.1 Factors Predisposing Patients to AF
• Hypertension (BP >140/90 mm Hg): RR 1.2–1.5 ▪ Pre-hypertension (sBP 130–139 mm Hg): RR 1.3 ▪ Increased pulse pressure: RR 1.3 per 20 mm Hg • Valvular heart disease: RR 1.8–3.4 • LV systolic dysfunction: RR 4.5–5.9 ▪ Diastolic dysfunction: RR 3.3–5.3 ▪ Hypertrophic cardiomyopathy: RR 4–6 • Diabetes: RR 1.4–16 • Thyroid dysfunction: RR 3–6 ▪ Subclinical hyperthyroidism: RR 1.4 | • Obesity: RR 1.4–2.4 • Alcohol consumption (≥36 g/day): RR ~1.4 • Obstructive sleep apnea: RR 2.8–5.6 • Physical activity (lifetime >1500 h): RR 2.9 • Familial and genetic (AF in ≥1 parent): RR 1.85 • Congenital heart disease • Chronic kidney disease: RR 1.3–3.2 • Inflammation: RR 1.5–1.8 • Pericardial fat: RR 1.3–5.3 • Tobacco use: RR 1.5–2.1 |
○Clinical evaluation
▪ Define the duration and frequency of episodes.
• Date of first symptomatic attack or AF discovery
• Onset of current episode
• Clinical classification (Categorize the patient by the most frequent presentation.)
▪ Define the presence and nature of symptoms.
• Palpitations, dyspnea, fatigue, effort intolerance and pre-syncope predominate, but 21% are asymptomatic.
• Symptoms are usually secondary to the tachycardia and generally are alleviated with adequate rate control.
▪ Identify precipitating factors.
• Caffeine, exercise, alcohol, sleep deprivation, and emotional stress.
• Sleep or after a large meal (vagal-mediated AF)
▪ Review past evaluations and treatments.
▪ Assess the presence of underlying heart disease or other reversible conditions
○Classification of AF-related symptoms
▪ Canadian Cardiovascular Society Severity of Atrial Fibrillation (CCS-SAF)
• Class 0: Asymptomatic with respect to AF.
• Class 1: AF has a minimal effect on quality of life (QOL)
▫ Minimal or infrequent symptoms.
• Class 2: AF has a minor effect on QOL
▫ Mild awareness of symptoms or rare episodes of paroxysmal AF.
• Class 3: AF has a moderate effect on QOL.
▫ Awareness of symptoms on most days (persistent)
▫ More common episodes (every few months) or more severe symptoms (paroxysmal)
• Class 4: Symptoms of AF have a severe effect on QOL.
▫ Unpleasant symptoms (persistent) or frequent/highly symptomatic episodes (paroxysmal)
▫ Syncope or heart failure due to AF
▪ European Heart Rhythm Association (EHRA) score
• Class I: No symptoms
• Class II: Mild symptoms – normal daily activity not affected
• Class III: Severe symptoms – normal daily activity affected
• Class IV: Disabling symptoms – normal daily activity discontinued
12-Lead ECG
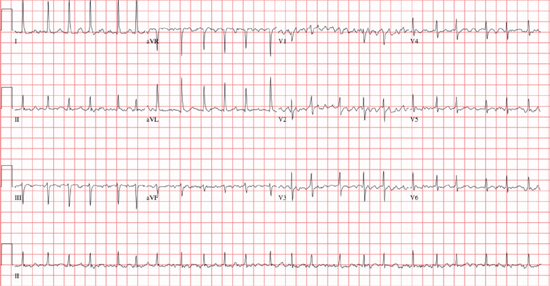
○ Rate: The atrial rate is usually 350–600 bpm.
▪ Due to multiple (~5–6) microreentrant circuits within the atria
○ Rhythm: The ventricular response is irregularly irregular (usually 100–180 bpm).
▪ <60 bpm: Slow ventricular response
• Due to intrinsic conduction abnormalities, or medications
▪ 70–110 bpm: Controlled ventricular response
• Due to intrinsic conduction abnormalities, or medications
▪ >120 bpm: Rapid ventricular response
• Hyper sympathetic state (generally rates >150 bpm): Drugs, stress, myocardial ischemia, pain, anxiety, infection, hypotension, anemia, thyrotoxicosis, hypoxemia, or hypoglycemia
• Pre-excitation (especially if rate >200 bpm)
▫ The ventricular rate is dependent on the refractory period of the AP.
○ P wave: There are no distinct P waves, but merely an undulating baseline (fibrillatory waves).
○ QRS: This is a narrow complex unless an aberrancy or bundle branch block [BBB]
○ Other: An ST segment shift is common, but only 1/3 have significant coronary disease.
Other Investigations
○Essential investigations
▪ Laboratory
• Complete blood count, electrolytes, renal function, hepatic function, thyroid function
▪ Electrocardiogram (ECG)
• P-wave duration and morphology (if in sinus), chamber hypertrophy, evidence of myocardial infarction
• R-R, QRS, and QT intervals: Baseline assessment prior to the initiation of antiarrhythmic drugs (AADs)
▪ Transthoracic echocardiogram (TTE)
• Structural heart disease (valvular pathology, cardiomyopathy, congenital heart disease)
• LA size, LV hypertrophy
○Additional testing
▪ 6-minute walk
• Adequacy of rate control
▪ Exercise stress test
• Exclude active ischemia prior to the initiating class Ic AADs in those at risk of ischemic heart disease.
• To assess the adequacy of rate control, AF-related symptoms, and to diagnose exercise-induced AF
▪ 24-hour Holter monitor or event recorder
• To confirm the diagnosis of AF
• To assess the adequacy of rate control
Management
Exclude Reversible Causes
○Myocardial disease: Myocardial infarction, myo-pericarditis
○Pulmonary disease: Embolism, pneumonia, sleep-disordered breathing
○Thyroid disease: 5.4% have subclinical hyperthyroidism; 1% have overt hyperthyroidism
○Acute alcohol and substance use
○Surgery: Cardiac (40%), thoracic (25%), orthopedic (15%), esophageal (5–10%)
▪ Peak incidence on day 2 (usually within 5 days)
▪ Prophylaxis: Oral β-blocker (class I) or amiodarone in high-risk patients (class IIa)
• Consider amiodarone for patients who are older, have peripheral vascular disease, valvular disease, chronic lung disease, or a large LA
Prevention of Thromboembolism
○Antithrombotic therapy is generally recommended for patients with AF unless contraindicated (see Table 9.2).
Table 9.2 Antithrombotic Therapy Recommendations for Patients with AF
| Recommended Therapy | |
Valvular AF • Mitral stenosis, mechanical prosthesis | Oral anticoagulation |
Hypertrophic cardiomyopathy | Oral anticoagulation |
Hyperthyroidism • Until a euthyroid state has been restored | Oral anticoagulation |
Non-valvular AF (estimate based on the CHADSVASc score) | |
0–1 point | ASA 81–325 mg or nothing |
≥2 points | Oral anticoagulation |
○Review the stroke risk scores for non-valvular AF (see Table 9.3).
Table 9.3 Stroke Risk for Patients with Non-Valvular AF
| Yearly Risk of Stroke | ||||||
| CHADS | CHADSVASc | Score | CHADS | CHADSVASc | ||
Clinical HF or LVEF <40% | 1 point | 1 points | 0 | 1.9% | 0% | |
Hypertension | 1 point | 1 points | 1 | 2.8% | 1.3% | |
Age ≥75 | 1 point | 2 points | 2 | 4% | 2.2% | |
Diabetes | 1 point | 1 points | 3 | 5.9% | 3.2% | |
Stroke/TIA/thromboembolism | 2 points | 2 points | 4 | 8.5% | 4% | |
Vascular disease (MI, PVD) | — | 1 points | 5 | 12.5% | 6.7% | |
Age 65–74 | — | 1 points | 6 | 18.2% | 9.8% | |
Sex (female) | — | 1 points | 7 | 9.6% | ||
8 | 6.7% | |||||
9 | 15.2% | |||||
HF: heart failure; LVEF: left ventricualr ejection fraction; MI: myocardial infarction; PVD: peripheral vascular disease; TIA: transient ischemic attack.
○Consider the risk of bleeding with warfarin therapy (see Table 9.4).
Table 9.4 Risk of Bleeding with Anticoagulation
| HASBLED Score | Outpatient Bleeding Risk Index | ||
Criteria | Criteria | ||
• Hypertension (sBP >160 mm Hg) • Abnormal renal function (Cr >200 μmol/L) • Abnormal liver function • Stroke • Bleeding • Labile INR • Elderly (age >65 years) • Drugs • Alcohol Risk of Major Bleeding • High risk defined for those with ≥3 points | 1 point 1 point 1 point 1 point 1 point 1 point 1 point 1 point 1 point | • Age ≥65y • History of stroke • History of GI bleed • Comorbidity ▪ Recent myocardial infarction ▪ Hct <30% ▪ Cr >1.5 mg/dL ▪ Diabetes Risk of Major Bleeding • Low (0 points = 0.8%/year) • Moderate (1–2 pt = 2.5%) • High (3–4 pt = 10.6%) | 1 point 1 point 1 point 1 point |
Cr: creatinine; GI: gastrointestinal; Hct: hematocrit; INR: international normalized ratio.
○When choosing an antithrombotic agent, consider the assessment of risks vs. benefits (see Table 9.5).
Table 9.5 Risk/Benefit of Antithrombotic Agents
| Study | Stroke Risk Reduction vs. Placebo | Major Bleeding | Comment | |
ASA 80–325 mg daily | AFASAK, SPAF, EAFT, ATAFS, PATAF, WASPO ESPS II, LASAF, UK-TIA | 22% | 1.5%–2.0% yearly | |
ASA + Clopidogrel | ACTIVE A, ACTIVE W | 43% (28% RRR vs. ASA) | 2.0%–2.5% yearly | |
Warfarin (INR 2–3) | AFASAK, SPAF, BAATAF, CAFA, PATAF, WASPO, ATAFS, SPINAF, EAFT | 64% (43% RRR vs. ASA + Clopidogrel) | 2.5%–3.0% yearly | |
Dabigatran 110 mg bid | RE-LY | 71% (9% RRR vs. W) | 20% reduction vs. W | This dose is preferred in those ≥80 years or with eGFR 30–50 mL/min |
Rivaroxaban 20 mg die | ROCKET-AF | 70% (12% RRR vs. W) | No difference vs. W | Use 15 mg daily if eGFR 30–50 mL/min |
ARISTOTLE AVERROES | 73% (21% RRR vs. W) | 31% reduction vs. W | Use 2.5 mg bid if 2 of: • age ≥80 years • weight ≤60 kg • Cr ≥133 mmol/L/ | |
Dabigatran 150 mg bid | RE-LY | 81% (34% RRR vs. W) | No difference vs. W |
eGFR: estimated glomerular filtration rate; RRR: relative risk reduction; W: warfarin.
▪ Note: Dabigatran, rivaroxaban, and apixaban are associated with a 10% decrease in all-cause mortality, which is largely due to the 40%–70% reduction in hemorrhagic stroke and ~50% reduction in intracranial hemorrhage (ICH)
○Interrupting anticoagulation
▪ Bridge with low-molecular-weight-heparins/unfractionated heparin (LMWH/UFH) if mechanical heart valve, high risk of stroke, or planned interruption >7 days
▪ If short interruption and risk of stroke is not significantly elevated, it is generally acceptable to interrupt oral anticoagulation without bridging LMWH or UFH.
Control of the Ventricular Rate
○Multiple large, randomized trials (AFFIRM, RACE, STAF, PIAF, HOT CAFÉ, AF-CHF) have demonstrated that a strategy of rate control can be at least as effective as rhythm control in properly selected patients.
▪ In these studies there was no difference in overall mortality, morbidity, or quality-of-life between rate and rhythm control.
▪ The rhythm control groups had an increased rate of hospitalization (usually for cardioversion).
○Clinical factors that may favor a primary strategy of ventricular rate control
▪ Patient preference
▪ Persistent AF
▪ Recurrent AF despite attempts at rhythm control (AAD or ablation)
▪ Less symptomatic patients
▪ Older patients (age >65 years)
▪ Comorbidities that would limit the success of achieving sinus rhythm
▪ Resting heart rate <80 bpm (but <100–110 bpm is reasonable if asymptomatic and normal LV function)
▪ Alternate targets
• 24-hour average heart rate <100 bpm
• Heart rate <110 bpm on 6 minute walk
• Heart rate <110% age-predicted maximum on exercise stress test
○ Choice of agent
▪β-blockers and calcium-channel blockers are generally regarded to be equally efficacious, although emerging evidence suggests a relatively greater survival benefit with BB.
•β-blockers are better rate-control agents (lower heart rate at rest/exercise) with no change or decreased exercise capacity.
• ND-CCBs are less effective rate-control agents (lesser reduction in heart rate on exertion) but lead to an increase or no change in exercise capacity.
▪ Digoxin is considered second line due to its inability to control HR during exertion or stress.
• As a single agent digoxin is ineffective at controlling ventricular rate in all but the sedentary elderly. The combination of digoxin and β-blockers is more effective than combinations of digoxin and ND-CCB, because of a synergistic effect on the AV node (AVN) (digoxin working well at rest with high vagal but low adrenergic tone, and β-blockers work well with stress with high adrenergic tone and vagal withdrawal).
○ AVN ablation after permanent pacemaker implanation
▪ Indications: Rapid ventricular rate despite medical therapy or significant medication-related side effects
▪ Outcome: Improves cardiac symptoms, quality of life, and healthcare utilization
▪ Limitations: Loss of AV synchrony (persistent symptoms in hypertrophic cardiomyopathy [HCM], restrictive cardiomyopathy [RCM], or hypertensive heart disease)
▪ Note: Consider biventricular pacing in the presence of impaired baseline LV function (e.g., LVEF <40%–50%).
Rhythm Control
○Pharmacotherapy to maintain sinus rhythm may be preferred with:
▪ First episode of AF or paroxysmal AF
▪ AF due to a reversible cause
▪ Highly symptomatic AF
▪ Patients of a younger age (<65 years of age)
▪ No previous antiarrhythmic drug (AAD) failure
▪ Patient preference
Cardioversion
○Cardioversion-related thromboembolism
▪ Risk does not differ between modalities (electrical/direct-current cardioversion [DCCV] or pharmacologic).
▪ Risk is decreased with adequate anticoagulation prior to cardioversion (<1% vs. 6.1% without anticoagulation).
▪ Risk is lowest in the first 12 hours after AF onset (0.3% vs. 0.7% after 12–48 hours).
• However, 60% of patients will spontaneously convert within 24 hours.
▪ Risk of stroke is highest in the first 3–10 days post cardioversion due to atrial stunning.
• Irrespective of modality, the patient requires 4 weeks of anticoagulation post cardioversion.
○Electrical or synchronized DCCV
▪ More effective and preferred for unstable patients
▪ Consider starting at higher energy outputs (e.g., > 150 J) in order to limit total energy exposure.
▪ Pre-treatment with AAD may enhance the success of DCCV
• SAFE-T trial: Placebo (68% acute success), amiodarone (72%), sotalol (73%)
• Other options: Flecainide, ibutilide, propafenone
•β-blockers and verapamil may reduce subacute recurrence only
○Pharmacologic cardioversion
▪ More likely to be successful if the AF is recent in onset; however, the success is always inferior to DCCV.
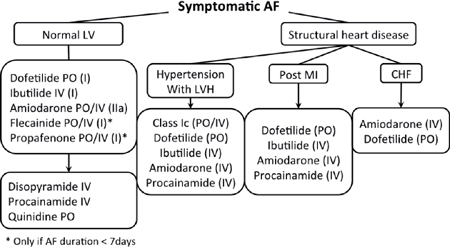
• Ibutilide: 1 mg IV infusion over 10 minutes (30%–45% rate of conversion)
• Procainamide: 1 g in 125 cc NS over 30 min IV (30% rate of conversion)
• Propafenone: 450 mg (<70 kg) or 600 mg PO (>70 kg) (50%–80% rate of conversion)
• Flecainide: 200 mg (<70 kg) or 300 mg (>70 kg) (50%–80% rate of conversion)
• Amiodarone: 150 mg IV bolus then infusion (30%–50% rate of conversion)
▪ A β-blocker or ND-CCB (diltiazem or verapamil) should be given before administering a class I AAD (i.e., procainamide, propafenone, flecainide), because these agents have can potentially slow the atrial rate, resulting in rapid conduction across the AVN (e.g., paradoxical increase in the ventricular rate).
Maintaining Sinus Rhythm Post Cardioversion
○Long-term anti-arrhythmic therapy
▪ The goal of AAD therapy is to decrease the frequency, severity, and duration of AF episodes as well as alleviate the associated symptomatology.
• AF recurrence while taking an AAD is not indicative of treatment failure and does not always necessitate a change in AAD therapy.
• Anticipated efficacy
▫ CTAF: 69% sinus at 16 months with amiodarone; 39% with sotalol or propafenone
▫ AFFIRM: 62% sinus at 1 year with amiodarone; 23% on class I agents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


