Chapter 27
Atherosclerotic Risk Factors
Smoking
Esther S.H. Kim, Heather L. Gornik
Tobacco smoking continues to have a devastating impact on public health and is a critical modifiable risk factor for atherosclerosis, including coronary artery disease (CAD) and peripheral artery disease (PAD).
Epidemiology
In the United States, it is estimated that during the period 2000-2004, 443,000 deaths each year were attributed to cigarette smoking, with 128,000 of those deaths due to cardiovascular disease and 161,000 due to malignant neoplasm (Fig. 27-1).1 The economic costs of smoking in the United States are estimated at $193 billion annually, with $97 billion in lost productivity and $96 billion in health care expenditures.1 Despite these alarming statistics, the estimated U.S. consumption of cigarettes in 2006 was 371 billion pieces,2 and cigarette smoking remains the leading preventable cause of disease and death in the United States.3
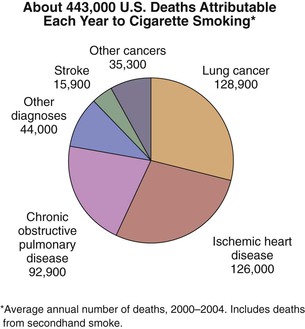
Figure 27-1 Estimated annual number of deaths in the United States attributable to cigarette smoking by specific cause of death. Data shown are based on the period 2000-2004. (Source: Centers for Disease Control and Prevention: Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep 57:1226-1228, 2008.)
Prevalence
The prevalence of cigarette smoking has decreased in the past 10 years, but 1 in 5 American adults aged 18 years and older, an estimated 45.3 million people, still consider themselves current smokers.3 According to data from the 2010 National Health Interview Survey and the 2010 Behavioral Risk Factor Surveillance System survey, smoking prevalence is higher among men (21.5%) than among women (17.3%), with the highest prevalence among adults aged 25 to 44 years (22.0%) and 45 to 64 years (21.1%).3,4 Non-Hispanic Indians/Alaska Natives have the highest prevalence, followed by non-Hispanic whites and non-Hispanic blacks (31.4% vs 21.0% vs 20.6%). The prevalence of cigarette smoking is also related to socioeconomic factors; smoking is more common among those living below the poverty level than above the poverty level (28.9% vs 18.3%) and is associated with lower level of education attained.3 Among Americans who smoke, approximately 69% of smokers want to quit completely and 52% of smokers had attempted to quit in 2010.4
In addition to socioeconomic factors, there may also be a genetic influence on smoking behavior and nicotine dependence. A genetic variant of the nicotinic acetylcholine receptor gene cluster on chromosome 15q24 was identified; it appears to have an effect on the quantity of cigarettes smoked per day and the risk for development of PAD in smokers of European descent.5 These results further support the established biologic relationship between smoking and arterial disease,6 but the threshold beyond which this relationship occurs is unknown.
Tar and Nicotine Content
Low-yield cigarettes that deliver less tar or nicotine in standardized machine measurements have been marketed by cigarette manufacturers as “light” or “ultralight” brands,7 but the existence of a nicotine or tar dose-response relationship is controversial with regard to risk of cardiovascular disease, and studies to date have provided conflicting results.8 One large epidemiologic study of more than 51,000 participants who were observed for 6 years found significantly fewer CAD-related deaths in participants who smoked cigarettes of low nicotine and tar content compared with those who smoked cigarettes of high nicotine and tar content (2.0-2.7 mg nicotine, 25.8-35.7 mg tar; 1007.5 deaths), with a mortality ratio of 0.83.8 Similarly, another prospective epidemiologic study consisting of 56,255 men observed for an average period of 13 years found that a 15-mg decrease in tar yield per cigarette resulted in a relative mortality of 0.77 for coronary heart disease and 0.86 for stroke.9 Two case-control studies have reported similar findings, with one study finding that a 5-mg decrease in tar yield resulted in a 13% reduction in cardiovascular disease risk10 and another finding that the risk of nonfatal myocardial infarction (MI) was 13% lower in people who smoke cigarettes with average tar yields of 7.5 mg versus those who smoke cigarettes with average tar yields of 13.3 mg.11 Importantly, all of these studies noted that smokers of low-tar cigarettes had greatly increased rates of cardiovascular events compared with nonsmokers.8–11
In contrast to the results of these studies, several studies found no relationship between nicotine and tar content and the risk of cardiovascular disease. Data from a male subset of the Framingham Heart Study showed that smokers of filtered cigarettes did not have lower rates of coronary events than smokers of unfiltered cigarettes.12 In addition, two case-control studies of the impact of nicotine and carbon monoxide content of cigarettes on incident MI in men13 and women14 found that although the risk of MI increased with the number of cigarettes smoked, the risk did not vary according to the nicotine or carbon monoxide content. Finally, a case-control study of a subset of data from the second Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2) trial,15 consisting of 916 patients with acute MI and 1106 controls, found that although the relative risk of acute MI increased with the number of cigarettes smoked daily, there was no relationship between acute MI and the tar yield of cigarettes smoked (relative risk compared with nonsmokers: 3.8, 4.3, 3.2, and 3.7 for tar yields of <10, 10-15, >15-20, and >20 mg, respectively).16 Whereas it is unclear whether low-tar cigarettes are less detrimental than high-tar cigarettes in terms of cardiovascular risk, it is obviously certain that “no tar” (i.e., no smoking) is the best option for prevention of cardiovascular events.
Biology
The pathobiology of cigarette smoking has been extensively studied. Cigarette smoking mediates its adverse cardiovascular effects through deleterious effects on the artery wall, particularly the endothelium, along with effects on sympathetic tone, metabolism, and the coagulation and fibrinolytic systems (Box 27-1).17–21 Despite extensive scientific knowledge, the precise component or components of cigarette smoke that lead to cardiovascular events is not known, although it is clear that both nicotine and carbon monoxide exposure are central.19,22–24
Endothelial Effects
Cigarette smoking is associated with endothelial cell damage and altered endothelial function.17 Studies of umbilical artery samples derived from infants born to smoking mothers compared with nonsmoking mothers have shown extensive distortion of the normal arterial intima, including subendothelial edema, widening of intercellular junctions, distention of the endoplasmic reticulum, and thickening of the basement membrane.25 Cigarette smoking increases platelet and leukocyte adhesion to the endothelial cells and increases permeability of the endothelial surface to fibrinogen.17,23,26,27 Cigarette smoking alters normal endothelial cell function, including decreased nitric oxide bioavailability and impaired vascular tone.28,29 Cigarette smoking is also associated with increased endothelial production of endothelin-1, a potent vasoconstrictor.30 Even brief secondhand smoke exposure has been shown to cause vascular injury in healthy volunteers, including increased circulating markers of endothelial injury, impairment of chemotaxis of circulating endothelial progenitor cells, and impaired flow-mediated vasodilatation of the brachial artery.31 The effect of cigarette smoking on endothelial function is synergistic with the effect of hyperlipidemia29 and may be ameliorated with statin treatment or antioxidants.32–34 Endothelial dysfunction associated with smoking is also reversible with smoking cessation.28 Cigarette smoking is a source of oxidative stress that potentiates endothelial dysfunction and is an important element of atherogenesis.29,34–36
Hematologic Effects
In addition to its vascular effects, cigarette smoking induces a procoagulant state associated with abnormalities of platelet function, coagulation, and fibrinolysis. The hematologic effects of smoking may account for the epidemiologic association of tobacco and atherothrombotic events, especially MI. Smokers have increased platelet aggregation compared with nonsmokers, and the addition of nicotine to in vitro platelet assays has been associated with increased aggregation.37–39 Smoking has been associated with multiple potentially prothrombotic abnormalities of the coagulation cascade, including fibrinogen and factor VII,21,40,41 along with increased tissue factor expression within atherosclerotic plaques in animal models.42 Smoking is associated with abnormalities of the fibrinolytic system, the body’s endogenous mechanism of anticoagulation, including impaired release of tissue plasminogen activator.43,44 Smoking increases blood viscosity in a dose-dependent manner that is reversible with smoking cessation.45,46
Physiologic Effects
Cigarette smoking leads to additional adverse physiologic and metabolic effects that augment its detrimental impact on cardiovascular health. Smoking causes nicotine-mediated increase in sympathetic tone, which leads to increased heart rate, blood pressure, and myocardial oxygen demand along with peripheral vasoconstriction.47–49 Smokeless tobacco, in the form of oral snuff, has been shown to have a similar effect on heart rate, blood pressure, and catecholamine levels in healthy volunteers.50 Smokeless tobacco is associated with increased and more sustained blood levels of nicotine compared with cigarette smoke.51 Smoking increases markers of inflammation, including total white blood cell count19,40 and C-reactive protein,52 and is associated with lower high-density lipoprotein and increased low-density lipoprotein, total cholesterol, and triglyceride levels compared with those of nonsmokers.18,19 These effects are at least partially reversible with smoking cessation.40,52 An association between smoking and insulin resistance has recently been identified, and it has been proposed that smokers most susceptible to the metabolic and cardiovascular consequences of smoking are those with coexisting insulin resistance.53–55
Relationship with Peripheral Arterial Disease
Smoking is a known contributor to the development of coronary atherosclerosis, but it is an even more powerful risk factor for the development of PAD. In fact, smoking has been shown to be twice as likely to cause PAD than CAD,56 and this association has been reproduced in several large epidemiologic studies. The earliest large epidemiologic study was the Framingham study, which observed 5183 men and women who were free of intermittent claudication at the beginning of the observation period for the development of intermittent claudication during 16 years of follow-up.6 When the cohort was categorized into smokers and nonsmokers, smokers developed intermittent claudication at twice the rate of nonsmokers. This relationship was dose dependent, with heavy smokers (those smoking more than 20 cigarettes per day) at highest risk for development of intermittent claudication.6 Continued follow-up of this cohort to 26 years confirmed that the twofold risk was consistent even to advanced age.57 These findings have been confirmed in other large epidemiologic studies; smoking increased the risk for development of PAD anywhere from twofold to sixfold,58–60 with heavier smokers (measured by higher plasma cotinine concentrations61) at highest risk for development of the disease.61,62 The estimated fraction of PAD attributable to smoking is as high as 76%.62
Many of the PAD-related events in the studies discussed were overrepresented by events occurring in men, but smoking also confers a potent risk for PAD in women as demonstrated by the largest epidemiologic study of the effects of smoking on incident symptomatic PAD in women.63 The Women’s Health Study included 39,825 women with no cardiovascular disease observed prospectively for a median of 12.7 years. During this period of follow-up, smoking had a potent, graded relationship with incident symptomatic PAD such that the multivariate adjusted hazard ratio was 3.14 (2.01-4.90), 8.93 (5.02-15.89), and 16.95 (10.77-26.67) for the development of symptomatic PAD in former smokers, women who smoked less than 15 cigarettes per day, and women who smoked 15 or more cigarettes per day compared with women who were lifetime nonsmokers. Stratified by pack-years of smoking, those with less than 10, 10 to less than 30, and 30 or more pack-years had multivariate adjusted hazard ratios of 2.52 (1.49-4.25), 6.75(4.33-10.52), and 11.09 (6.94-17.72), respectively.63
Special note must be made of the relationship between smoking and thromboangiitis obliterans or Buerger’s disease (see Chapter 79) and abdominal aortic aneurysm (see Chapter 130). Buerger’s disease occurs almost exclusively in smokers, and smoking cessation has been shown to be the only effective treatment of this condition.64 Smoking is the most important modifiable risk factor for the development of abdominal aortic aneurysm,65 and ongoing smoking is associated with more rapid aneurysm expansion during ultrasound follow-up.66
Effect of Smoking Cessation
Whereas smoking has become an established risk factor for the development of atherosclerosis, there is some evidence that it continues to increase the biologic progression of atherosclerosis even after smoking cessation. Some studies, particularly of carotid intima-media thickness, suggest that cigarette smoking may be an independent predictor of atherosclerosis progression.67,68 The Atherosclerosis Risk in Communities (ARIC) study observed 10,914 participants for longitudinal assessment of the relationship between smoking exposure and change in atherosclerosis, as measured by carotid intima-media thickness, during a period of 3 years.68 After adjustment for demographic variables and cardiovascular risk factors, current smokers had a 50% increase in the progression of intima-media thickness relative to never-smokers (mean progression of 43.0 µm versus 28.6 µm for current smokers versus never-smokers). Former smokers had a 25% increase in the progression of atherosclerosis relative to never-smokers,68 and after adjustment for the number of pack-years smoked, there was no difference in the progression of atherosclerosis between current and former smokers. The authors of the ARIC study indicated that this result suggests that smoking causes an irreversible and cumulative effect on the biology of atherosclerosis progression.68 Within the ARIC cohort, environmental tobacco smoke exposure was also associated with increased progression of carotid intima-media thickness during the follow-up period.
The findings of the ARIC study are supported by an epidemiologic study of 744 older men living in western Australia.69 Smoking was a primary risk factor associated with the development of PAD (current smoking odds ratio of 3.9 vs former smoking odds ratio of 2.0), with 32% of PAD attributable to current smoking. Interestingly, a further 40% of PAD was attributable to a history of past smoking among men who did not smoke currently.69 This led the investigators to conclude that previous smoking among patients who quit continues to increase the risk of PAD.
There are conflicting data to suggest that smoking cessation decreases the risk for development of PAD. In a cross-sectional study of 2334 older (≥60 years) Chinese subjects who were screened for PAD by the Rose Claudication Questionnaire and the ankle-brachial index,70 the odds ratio for PAD among current smokers versus never-smokers was 1.54 and 1.28 for former smokers versus never-smokers. Similar to the results of the ARIC study,68 there was a dose-dependent relationship between the number of cigarettes smoked and risk of PAD. However, in contrast to the ARIC68 and the western Australia69 cohorts, the Chinese study found that smoking cessation dramatically reduced the risk of PAD, such that cessation for more than 10 years almost eliminated the excess risk associated with smoking.70 This decrease in the risk of PAD with smoking cessation was reproduced in a study of 2517 Korean men aged 50 years or older.71 As expected, there was a graded relationship between smoking and PAD such that compared with never-smokers, the adjusted odds ratio of PAD for smokers of 0.1 to 20.0, 20.1 to 40.0, and more than 40.0 pack-years were 2.15 (1.06-4.38), 2.24 (1.08-4.65), and 2.93 (1.41-6.09), respectively. However, there was also an inverse relationship between years since tobacco cessation to risk of PAD such that the adjusted odds ratio of PAD for 11 to 20 and 21 or more years of smoking abstinence were 0.41 (0.19-0.86) and 0.49 (0.24-0.98) compared with current smokers.71
Although there may be some debate as to whether smoking cessation decreases the risk for development of PAD, it has been well established that continued smoking worsens the signs and symptoms of PAD. This was well demonstrated in a study that repeated ankle-brachial indices in 10 older (63 ± 10 years) chronic smokers with PAD (ABI = 0.64 ± 0.14) on smoking and nonsmoking days.72 The ankle-brachial index on the smoking day was significantly lower than on the nonsmoking day (0.55 ± 0.11 versus 0.64 ± 0.14; P = .008) owing to lower ankle systolic blood pressures.72 These results provide evidence of the acute physiologic effects of smoking on the peripheral circulation. The chronic arterial effects of tobacco have been demonstrated in multiple studies showing that current smokers with PAD have more severe claudication and inferior cardiorespiratory parameters at peak exercise than do former smokers.73–75 In a study of 38 current smokers and 100 former smokers with claudication who were recruited to undergo exercise testing, current smokers had lower ankle-brachial indices, earlier time to onset and to maximal pain, and longer time to pain relief after exercise.74 In addition, current smokers had lower peak oxygen uptake, lower heart rate, higher ventilation, and higher diastolic blood pressures than former smokers (all relationships compared with former smokers <.05). Of note, the finding of worsened exercise performance in current smokers versus former smokers was independent of the resting ankle-brachial index.74
When current and former smokers were compared with nonsmokers in a study that exercised patients with intermittent claudication using the 6-minute walk test, nonsmokers walked significantly farther (P < .05) than either current or former smokers (413 ± 14 m vs 352 ± 7 m and 370 ± 7 m, respectively), but there was no difference in distance walked between smokers and former smokers.76 Former smokers in this study self-reported abstinence for at least 12 months. These results again suggest that the effects of cigarette smoking on the biology of atherosclerosis in the peripheral vasculature may be only partially reversible.
Benefits of Smoking Cessation
The clinical beneficial effects of smoking cessation among patients with PAD include improvement in claudication,73,74 modest improvement in the ankle-brachial index,77 reduction in the likelihood of amputation,78 improvement in the patency rates of arterial bypass grafts,79 and improvement in overall survival.80
Ankle Pressure
One of the first studies to examine the relationship between smoking cessation and improvement in ankle-brachial indices and maximum treadmill walking distance included 62 patients (124 limbs) with intermittent claudication.77 Ankle-brachial indices were recorded every 3 months at rest and after treadmill exercise; participants were categorized into those who continued to smoke after the first test or quit smoking after the first test and continued to abstain until after the last test. Ankle-brachial indices were unchanged in the group who continued to smoke (0.82 ± 0.20 at test 1, 0.78 ± 0.19 at test 3; P = .13) but significantly improved in those who quit smoking after the first test (0.71 ± 0.18 at test 1, 0.82 ± 0.19 at test 3; P = .001). Similarly, maximum treadmill walking distance did not change among those who continued to smoke (230.4 ± 96.3 m at test 1, 254.2 ± 102.6 m at test 3; P = .179) but significantly increased (214.7 ± 87 m at test 1, 300.9 ± 129.9 m at test 3; P = .018) in those who quit smoking.77
Amputation Rate and Graft Patency
Continued tobacco smoking increases the likelihood of amputation among patients with PAD.81 Amputation rates are significantly associated with smoking intensity, as was shown in a study of 125 post-revascularization patients who were characterized as either moderate smokers (<15 cigarettes daily) or heavy smokers (≥15 cigarettes daily).81 During the subsequent 3 years, the amputation rate was 2% in moderate smokers and 21% in heavy smokers (P < .001).81 The alarming rate of amputation in heavy smokers after revascularization may have been related to increased graft failure in patients who continue to smoke even after surgery. A meta-analysis of 19 studies (10 prospective, 9 retrospective) of the effects of smoking and smoking cessation on lower extremity bypass patency rates showed a twofold to threefold increase in the risk of graft failure among patients who smoked versus nonsmokers.79 Furthermore, heavy smokers had decreased patency compared with moderate smokers, and patients who quit smoking from the time of bypass surgery had patency rates similar to those of never-smokers.
Survival
Finally, smoking cessation is associated with improved survival among patients with PAD, as has been demonstrated in multiple epidemiologic studies.75,80 One of the first studies to investigate the survival benefit of smoking cessation compared with continued smoking among patients with severe, symptomatic PAD found that patients who quit smoking had twice the survival rate at 5 years compared with those who continued to smoke (66% vs 36%; P < .01).80 Similarly, a study of 343 smokers and former smokers (quit within 6 months of study enrollment) with intermittent claudication who were observed for 10 years found a significant improvement in the rates of MI (11% vs 53%; P < .05) and cardiac death (6% vs 43%; P < .05) among former smokers compared with active smokers, respectively.75 In contrast to the previous study, however, the differing rates of survival at 10 years of follow-up (46% in smokers vs 82% in former smokers; P = .076) were not statistically significant until after 1 year, suggesting that the effect of smoking cessation on survival may not be immediately apparent.75
The Nurses Health Study reported the effects of smoking cessation on mortality among 104,519 female participants.82 This prospective observational study with 24 years of follow-up, including 12,483 deaths, found that compared with never-smokers, current smokers had a twofold to threefold risk of all-cause mortality (hazard ratio, 2.81), and the risk increased with the number of cigarettes smoked.82 There was a 13% reduction in the risk of all-cause mortality for former smokers 5 years after quitting compared with continued smoking, and the excess risk for all-cause mortality for current smokers decreased to the level of never-smokers 20 years after quitting. There was a more rapid decline in the risk for vascular disease–related mortality in the first 5 years after quitting compared with other causes of death, including lung disease and cancer, such that 61% and 42% of the mortality benefit of quitting for coronary and cerebrovascular disease, respectively, occurred in the first 5 years of smoking cessation. Finally, 64% and 28% of deaths among current and former smokers, respectively, were attributable to cigarette smoking.82
Smoking Cessation in Clinical Practice
Based on the U.S. Public Health Service clinical practice guidelines for treatment of tobacco use and dependence,83,84 a useful acronym to aid in the treatment of tobacco dependence in smokers willing to quit is the 5 A‘s: Ask, Advise, Assess, Assist, and Arrange.83 An algorithmic approach to smoking cessation using the 5 A‘s is shown in Figure 27-2.85 For the patient who has expressed interest in tobacco cessation in the next 30 days, a quit date should be noted and a follow-up visit should be arranged soon after the quit date to assess the patient’s success at cessation. Behavioral support in the form of educational materials, referral to online or group counseling programs, and practical counseling by the physician should be included during the visit when the patient expresses a desire to quit. Practical counseling includes encouragement of other smokers in the patient’s household to join in the quit attempt and helping the patient to identify triggers for smoking and how to overcome them.84 First-line pharmacologic therapies include nicotine replacement, bupropion, and varenicline. All three agents have been shown to be more effective for smoking cessation compared with placebo, and prescribing practices should be individualized to each patient on the basis of contraindications and potential side effects. The effectiveness of each pharmacologic therapy, suggested dosing, side effect profile, and contraindications are described in detail in the following section and Tables 27-1 and 27-2.
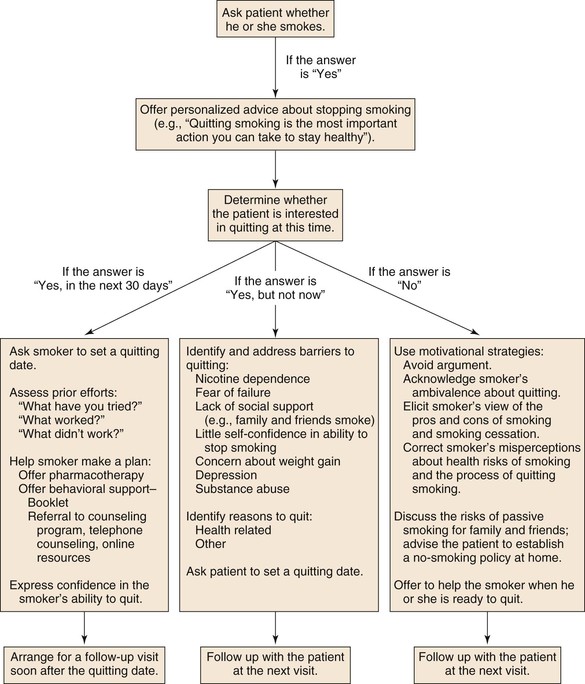
Figure 27-2 An algorithmic approach to smoking cessation treatment using the 5 A‘s.83 (Modified from Rigotti NA: Clinical practice. Treatment of tobacco use and dependence. N Engl J Med 346:506-512, 2002.)
Table 27-1
Nicotine Replacement Therapy for Smoking Cessation
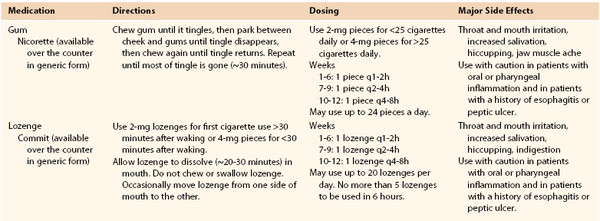
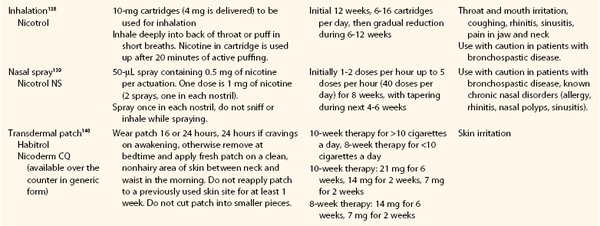
From Guideline Update Panel: Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care 53:1217-1222, 2008.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


