Chapter 28
Atherosclerotic Risk Factors
Diabetes
Matthew G. Nayor, Joshua A. Beckman
Based on a chapter in the seventh edition by Peter Sheehan
Diabetes is characterized by chronic hyperglycemia resulting either from a lack of insulin production (type 1) or from insulin resistance (type 2). In the last several decades, an alarming rise in the global prevalence of diabetes has been seen. The cost to the health care system is enormous because the medical expenditures of people with diabetes are two to three times higher than those of the rest of the population.1
The harmful consequences of diabetes are primarily vascular and are routinely divided into microvascular and macrovascular categories. The most important microvascular complications are retinopathy and nephropathy; people with diabetes have a 20-fold increased relative risk of blindness and a 25-fold higher relative risk of end-stage renal disease compared with people without diabetes. Macrovascular disease is characterized by atherosclerosis.2 Diabetes is an important risk factor for the development and severity of all forms of atherosclerosis, including peripheral artery disease (PAD), coronary artery disease (CAD), and cerebrovascular disease (CVD). Most of the 230,000 diabetes-related deaths in the United States every year are due to cardiovascular disease.1 Diabetes also increases the risk of ischemic stroke two- to threefold and accounts for 60% of nontraumatic lower-limb amputations.3–7 These financial and physical costs are expected to increase in the next decades as the prevalence of diabetes continues to rise worldwide.
Epidemiology
Over the last several decades, the global prevalence of diabetes has steadily increased, with an estimated 366 million people worldwide currently diagnosed with the condition. This number is predicted to reach 500 million by 2030, which equates to an annual increase in diabetes prevalence that is 1.7 times faster than the annual growth of the world’s population. In the United States, 8.3% of the population, or 25.8 million people, have diabetes and 79 million people have prediabetes (characterized by insulin resistance).2 Although the prevalence of diabetes is shifting to a younger demographic as the overall population becomes more obese, the risk of diabetes continues to increase with age, and as many as 26.9% of all Americans over the age of 65 have diabetes.1 The risk is higher for non-Hispanic blacks and Hispanic Americans than for whites or Asian Americans.1
Lifestyle changes related to increasing industrialization and economic development appear largely responsible for the dramatic rise in diabetes prevalence. Chief among these are the increasing rates of obesity, which have been attributed to a sedentary lifestyle and a Western diet rich in high-calorie food. The prevalence of obesity in the United States is increasing at an alarming rate and directly corresponds to the number of new diagnoses of diabetes. In fact, from 2000 to 2005, the rate of obesity increased by 24%. During that same time period, the prevalence of diabetes in the United States increased from 12% to 16.3%.1 Recent data, however, point to the specific role of sugar consumption, independent of obesity and other types of food intake.8 Indeed, no other food type was associated with diabetes, whereas a direct relationship was found between the amount of sugar intake and diabetes risk.
Average medical expenditures for patients with diabetes are 2.3 times higher than for those without diabetes. In 2007, the total cost of diabetes in the United States was estimated to be $218 billion. Perhaps most profound is the relationship between diabetes and cardiovascular death, which accounts for the majority of diabetes-related deaths in the United States annually.1
Classification of Diabetes
Type 1
Type 1 diabetes is characterized by an absolute deficiency in insulin secretion and accounts for 5% to 10% of diabetes diagnoses.9 It results from cellular-mediated autoimmune destruction of the pancreatic β cells and requires both genetic and environmental factors to cause the disease state. Markers of immune destruction of the β cell are present in 70% to 90% of patients and can aid in the diagnosis. These include islet cell autoantibodies, autoantibodies to insulin, anti−glutamic acid decarboxylase antibodies, and autoantibodies to tyrosine phosphatase IA-2 and IA-2β.10
Typically, type 1 diabetes presents with acute hyperglycemia or ketoacidosis as the first disease manifestation. Type 1 diabetes (previously known as “juvenile-onset” diabetes) often presents in children and adolescents but can present at any age. It also frequently develops in patients who have other autoimmune diseases such as lupus, rheumatoid arthritis, and Hashimoto’s thyroiditis.9 As a result of the absolute deficiency in insulin secretion, patients with type 1 diabetes are reliant on insulin replacement therapy.
Type 2
Type 2 diabetes results from a combination of insulin resistance and inadequate compensatory insulin secretion. It accounts for 90% to 95% of diabetes cases.9 Although type 2 was previously referred to as either “adult-onset” or “non–insulin-dependent” diabetes, these terms are less accurate because many patients eventually require insulin treatment and because type 2 diabetes can develop at practically any age. The pathogenesis of type 2 diabetes is heterogeneous, with both environmental and genetic causes. Obesity is strongly related to insulin resistance and is the most important environmental factor. Although insulin resistance is clearly necessary for the development of type 2 diabetes, incomplete compensatory rise in insulin secretion (relative deficiency) must also be present for hyperglycemia to result. This concept was illustrated by DeFronzo et al, who demonstrated that plasma insulin response to ingested glucose increases progressively until the fasting glucose concentration reaches 120 mg/dL. Thereafter, increases in fasting glucose are associated with progressive decline in insulin secretion.11 The genetic predisposition to type 2 diabetes is also well defined. The lifetime risk of developing type 2 diabetes is 40% in individuals with one parent affected and 70% if both parents are affected.12 In type 2 diabetes, hyperglycemia tends to develop slowly, and the symptoms are therefore more subtle. These include polyuria, polydipsia, weight loss, and polyphagia. People with type 2 diabetes have varying levels of insulin resistance and deficiencies in insulin secretion, and titration of different medications is often necessary to achieve appropriate glycemic control.
Diabetes and Vascular Disease
Vascular disease is the most significant cause of morbidity and mortality in people with diabetes.3 Microvascular diseases, such as retinopathy, nephropathy, and neuropathy, are associated with a direct relationship between level of hyperglycemia and disease severity; thus these diseases are more prominent in type 1 diabetes, with its long duration of hyperglycemia exposure.3 On the other hand, macrovascular complications such as CAD, PAD, and CVD, although responsible for the majority of deaths in patients with diabetes, have a modest relationship to glycemia.13
Coronary Artery Disease
Diabetes is associated with a significantly increased risk of developing CAD, and patients with diabetes and CAD have been shown to have worse outcomes. People with diabetes account for 30% of patients presenting with acute coronary syndromes, although they make up only 8% of the general population.14 Furthermore, 75% of people with diabetes die of complications related to CAD.15 People with diabetes tend to present with CAD at a younger age than patients without diabetes. It is estimated that diabetes leads to clinically evident CAD as much as 15 years earlier than otherwise expected.16 Once diagnosed with CAD, persons with diabetes have a higher risk of cardiovascular death, recurrent myocardial infarction (MI), stroke, and coronary stent thrombosis.17 The risk is further increased in patients on insulin therapy, which likely serves as a marker of disease severity. In addition, following MI, the 1-month mortality rate is 58% higher in people with diabetes.18
Despite the increased rates of cardiovascular morbidity and mortality, asymptomatic diabetic patients do not require stress testing to determine whether there is silent coronary heart disease. In the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study, 22% of patients had silent ischemia.19 The strongest predictor for silent ischemia was diabetes duration. Despite the higher rate of silent ischemia, the DIAD study made clear that conducting stress testing in asymptomatic patients with diabetes to find coronary heart disease does not reduce cardiovascular outcomes beyond standard risk factor modification.20 The results were similar in the Do You Need to Assess Myocardial Ischemia in Type-2 diabetes (DYNAMIT) trial.21 Therefore evaluation of patients with diabetes undergoing noncardiac surgery does not differ from other patient populations and should follow the 2007 American College of Cardiology/American Heart Association (ACC/AHA) guidelines, which emphasize assessment of cardiovascular symptoms and functional capacity.22
Peripheral Artery Disease
The prevalence of PAD varies significantly based on the age of the population studied. In the National Health and Nutrition Examination Survey (NHANES), the prevalence of PAD in Americans older than 40 years was 4.3%.23 However, it was 19.8% in men 65 years and older in the German Epidemiological Trial on Ankle Brachial Index (GETABI).24 Targeted screening can more clearly identify a population at risk. In the PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) trial, nearly 7000 subjects were screened in primary care practices, provided they met one of the following criteria: age 70 years or greater or ages 50 to 69 years with a history of diabetes and/or smoking.25 Using these criteria, 29% of subjects were found to have PAD. In patients with diabetes, risk of PAD is increased by older age, duration of diabetes, and presence of peripheral neuropathy. In the Edinburgh Artery Study, the prevalence of PAD was 12.5% in patients with normal glucose tolerance test, 19.9% in patients with impaired glucose tolerance testing, and 22.4% in patients with diabetes.26 Using the ABI for diagnosis, another survey found the prevalence of PAD to be 20% in people 40 years and older with diabetes, nearly 5 times that expected in patients without PAD.7 The risk of PAD is also known to be higher in African Americans and Hispanic Americans with diabetes.5
Diabetes significantly increases both the incidence and severity of limb ischemia because of several associated factors.27 The distribution of the PAD is different in patients with diabetes compared with those without it. Patients with diabetes and PAD tend to have involvement of the more distal arteries, particularly the popliteal and tibial arteries, making limb-salvage revascularization more challenging.5,6 The neuropathy that often develops in people with diabetes presents several additional challenges. First, sensory neuropathy reduces the ability to avoid injury by decreasing normal sensation and withdrawal to pain. In addition, symptoms common to advanced disease may be less appreciated and may lead to delay in diagnosis.6 Diabetic peripheral neuropathy also leads to limited joint mobility (due to motor neuropathy), decreased proprioception and pain sensation (due to sensory neuropathy), and decreased sweating (due to autonomic neuropathy). The motor neuropathy fosters the formation of a swan-neck foot deformity, which greatly increases the pressure to the ball of the foot making ulceration more likely.28,29
As a result, diabetes is the most common cause of nontraumatic lower extremity amputation in the United States, accounting for 55% of amputation-related hospitalizations.30 For people 65 to 74 years old, the risk of amputation is increased more than 20-fold compared with those without PAD and diabetes.31 The combination of PAD and diabetes is of additional clinical importance given its association with cardiovascular events. Patients with both diabetes and PAD are at extremely high risk of adverse cardiovascular events. In the Heart Protection Study (HPS), the risk of cardiovascular event over a 5-year period was 10% for people with diabetes, 20% for those with PAD, and 30% for patients with both.32
Vascular Evaluation of Patients with Diabetes
The vascular evaluation of patients with diabetes differs little from evaluation of patients without diabetes and is well reviewed elsewhere in this text. One important diagnostic consideration, however, is the increased likelihood of noncompressible pedal vessels and subsequent falsely elevated ABI results in patients with diabetes. Diabetes does provide further challenges and requires additional evaluation for a comprehensive assessment, particularly regarding risk factor assessment and the evaluation of neuropathy.
The presence of neuropathy is an important risk multiplier not seen with other risk factors. Diabetic peripheral neuropathy is characterized by a symmetric sensorimotor polyneuropathy.33 It starts distally, moves proximally, and results in a typical “glove and stocking” distribution.34 Motor deficits are rare in the early stages of diabetic peripheral neuropaty. Burning, tingling, and shooting pains are frequently described and are typically worse at night.34 Of note, the degree of pain and subjective symptoms are not reliable indicators of sensory nerve damage. Therefore careful examination and monitoring of patients with diabetes are necessary because sensory loss can exacerbate foot ulceration and lead to unintentional injuries. Careful peripheral neurologic examination is recommended annually in patients with diabetes.35 Electrophysiologic testing is rarely necessary. The examination should focus on inspection of the extremities and feet for signs of skin change, hair loss, ulceration, or increased dryness. Full sensory and motor exam should then be performed with the addition of monofilament testing plus vibration sensation (using 128-Hz tuning fork), pinprick sensation, or ankle reflexes.35
Pathophysiology of Vascular Disease in Diabetes
Diabetes leads to increased atherosclerotic vascular disease by a number of mechanisms, including metabolic derangements, hypercoagulability, inflammation, vascular dysfunction, and neuropathy. These alterations result in a phenotypic change in the blood vessel from one of homeostasis to an atherogenic phenotype characterized by endothelial cell dysfunction, oxidative stress mediated by increased production of free radicals, and vascular smooth muscle dysfunction.
Dysmetabolism and Vascular Dysfunction
The cardinal metabolic derangements in diabetes are each associated with a wide variety of insults that attenuate the vasculature’s ability to maintain equilibrium and foster an environment permissive for the development of atherosclerosis. The two fundamental derangements include hyperglycemia and insulin resistance.
Both hyperglycemia and insulin resistance are associated with atherosclerosis. Increases in the rate of atherosclerotic events begin with modest increases in fasting glucose levels in the normal range. Insulin resistance, independent of hyperglycemia, is associated with atherosclerosis and predicts cardiovascular events.36 Indeed, a majority of patients with coronary heart disease have insulin resistance or frank diabetes. In the Euro Heart Survey performed in 110 medical centers in 25 nations, 4961 subjects with coronary artery disease but no known diabetes were enrolled, and a majority of these patients were subsequently found to have diabetes, impaired glucose tolerance, or impaired fasting glucose.37 The results have been replicated in non-European populations as well.38
The impact of diabetes on the vascular endothelium represents an important link between the dysmetabolism of diabetes and the atherosclerosis that causes the majority of morbidity and mortality. The vascular endothelium plays a fundamental role in vascular homeostasis, regulating vascular tone, platelet activity, leukocyte adhesion and diapedesis, and vascular smooth muscle cell migration and proliferation.39 The endothelium regulates vascular homeostasis through the elaboration of autocrines and paracrines that modulate the structure and function of vascular cells. The endothelium-derived vasodilator, nitric oxide (NO), is constitutively produced in healthy endothelial cells by endothelial nitric oxide synthase (eNOS). The production of NO is closely adjusted by a wide variety of chemical and biomechanical stimuli. In addition to its potent vasodilatory properties, NO reduces production of proinflammatory chemokines and cytokines through inhibition of inflammatory transcription factors, which subsequently limits platelet activation. In contrast, decreased bioavailability of NO enhances an environment of vascular injury and atherogenesis.40 NO bioavailability is reduced in basic investigations, animal models, and humans with insulin resistance and frank diabetes mellitus.41–44 Endothelial dysfunction, found in both hyperglycemia and impaired endothelial insulin signaling, may link insulin resistance to its heightened risk of atherosclerosis, MI, and death. Thus endothelial dysfunction participates in the development and progression of atherosclerosis and may facilitate its adverse sequelae.
Hyperglycemia impairs vascular function through an increase in the production of reactive oxygen species (ROS), oxidative stress, and consequent impairment in endothelial function.45 Hyperglycemia-induced ROS inactivates endothelium-derived NO.45 Reduced NO bioavailability fosters atherogenesis and predicts a heightened risk of cardiovascular outcomes.46,47 Through a variety of mechanisms, hyperglycemia increases ROS production and impairs endothelial function. Hyperglycemia increases mitochondrial generation of superoxide anion, leading to cellular mitogenic pathway activation including polyol and hexosamine flux, advanced glycation end products (AGEs), protein kinase C (PKC) activation, and nuclear factor κB (NF-κB)–mediated vascular inflammation.48,49 Indeed, ROS lead to upregulation and nuclear translocation of NF-κB subunit p65 and transcription of proinflammatory genes encoding for monocyte chemoattractant protein-1 (MCP-1), selectins, vascular cell adhesion molecule-1 (VCAM-1), and intracellular adhesion molecule-1 (ICAM-1). These events facilitate adhesion of monocytes to the vascular wall and translocation into the subendothelium with subsequent formation of foam cells (Fig. 28-1).
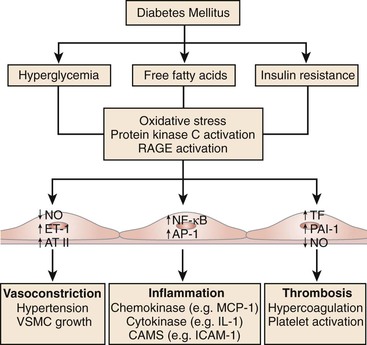
Figure 28-1 The metabolic abnormalities that characterize diabetes—particularly hyperglycemia, free fatty acids, and insulin resistance—provoke molecular mechanisms that alter the function and structure of blood vessels.
The second cardinal marker of dysmetabolism in diabetes is insulin resistance. Insulin resistance likely precedes the onset of hyperglycemia by many years. In diabetes, insulin resistance affects many tissues, including skeletal muscle, liver, adipose, and blood vessels. One possible mechanism by which insulin resistance can impair vascular function is the byproduct of the resistance on adipose tissue. Adipose is an important source of inflammatory mediators and free fatty acids (FFA).50 Obese patients with type 2 diabetes have increased plasma levels of free fatty acids and inflammatory markers.51 Impaired endothelial cell insulin signaling may also be salient. In mice, a loss of insulin signaling in the vascular endothelium leads to diminished endothelial nitric oxide synthase levels, endothelial dysfunction, expression of adhesion molecules, and atherosclerotic lesions.52 Another study confirmed the importance of endothelial insulin signaling by showing that genetic disruption of endothelial insulin receptor substrate 2 (IRS-2) reduces glucose uptake by skeletal muscle,53 whereas restoration of insulin-induced eNOS phosphorylation restored capillary recruitment as well as insulin delivery.53 These novel findings strengthen the central role of endothelium in obesity-induced insulin resistance, suggesting that blockade of vascular inflammation and oxidative stress may be a promising approach to prevent metabolic disorders. Notably, pharmacologic improvement of insulin sensitivity in patients with type 2 diabetes and metabolic syndrome is associated with restoration of flow-mediated vasodilation.54–56
The atherogenic effects of insulin resistance are also due to changes in lipid profile such as high triglycerides, low HDL cholesterol, increased remnant lipoproteins, elevated apolipoprotein B (ApoB), and small and dense LDL.57 Once circulating free fatty acids reach the liver, VLDL are assembled and made soluble by increased synthesis of apolipoprotein B. VLDL are processed by cholesteryl ester transfer protein (CETP), allowing transfer of triglycerides to LDL, which become small and dense and, hence, more atherogenic. Atherogenic dyslipidemia is a reliable predictor of cardiovascular risk, and its pharmacologic modulation may reduce vascular events in subjects with type 2 diabetes and metabolic syndrome.58–60
Platelet Dysfunction and Coagulation Cascade
Platelet dysfunction has also been shown to play a role in thrombosis, complicating atherosclerotic plaque rupture in diabetes. Glycoprotein Ib and IIb/IIIa expression is upregulated in diabetes, which leads to increased amounts of von Willebrand factor and platelet-fibrin interaction.61 Hyperglycemia also impairs calcium homeostasis, which alters calcium-dependent platelet aggregation and activation.62 Procoagulant factors (factor VIII, thrombin, and tissue factor) are increased and endogenous anticoagulants and fibrin inhibitors (thrombomodulin, protein C, plasminogen activator inhibitor 1) are decreased in a chronic hyperglycemic state.63–66 Diabetes therefore leads to increased platelet aggregation and a shift in favor of procoagulant factors of the thrombotic cascade. These alterations contribute to the propensity not only for atherosclerosis, but for pathologic plaque rupture resulting in acute coronary syndrome, ischemic stroke, and acute limb ischemia, which are known to be more common in people with diabetes.
Treatment of Patients with Diabetes And PAD
The two most important goals in the treatment of patients with PAD and diabetes are improving limb outcomes (i.e., improving claudication symptoms and preventing progression to critical limb ischemia) and decreasing morbidity and mortality from cardiovascular disease and stroke. An aggressive approach to risk factor modification and medical treatment is necessary to achieve both goals. Target-driven medical intervention can reduce the risk of cardiovascular by ≈50% in patients with type 2 diabetes.67 A sample treatment algorithm for patients with PAD and diabetes is available in Box 28-1.
Preventive Foot Care
Peripheral neuropathy, ischemia, and infection form the etiologic triad of diabetic foot complications.68 Commonly, diabetic foot ulcers and infections begin as small wounds that are not recognized and treated in the early stages because of symptoms may be masked by sensory neuropathy. Therefore careful screening and early intervention are important in preventing diabetic foot complications. The American Diabetes Association (ADA) recommends annual foot examination to identify high-risk conditions before complications develop.69 Proper foot care and hygiene are the hallmarks of preventive therapy. Areas of bony deformity or rubor should be treated with offloading and inserts as well as special footwear to prevent progression.68
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


