Chapter 47 Atheroembolism
Atheroembolism occurs when tiny fragments of an atherosclerotic plaque (in particular, cholesterol crystals) break off from a proximal artery and travel distally in the circulation, ending up in small arteries downstream from its origin. The consequence of this event is microvascular obstruction with tissue ischemia. The abdominal aorta is the most common origin for atheroembolism to the abdominal organs and lower extremities, but any artery with atheromatous disease may be a potential embolic source. End-organ targets include the brain, eye, heart, kidney, gastrointestinal tract, fingers, toes, and skin. The kidneys and skin are the two most common targets in many cases.1 In the setting of an elderly patient who develops sudden onset of pain and ischemia of the distal extremities and unexplained renal failure after an invasive angiographic procedure, atheroembolism should be high on the list of likely diagnoses.2
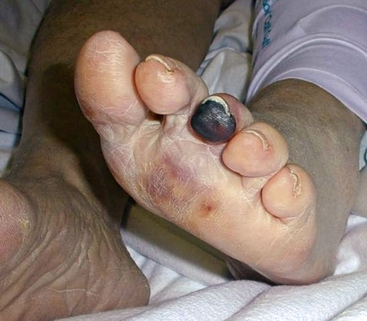
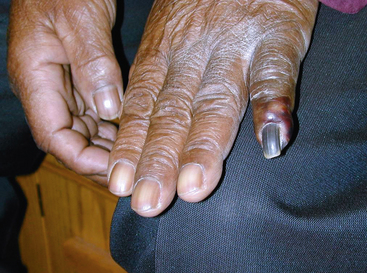
Atheroembolism can present in a number of distinct clinical syndromes (Box 47-1). The blue toe syndrome occurs when arteries to the distal parts of the feet and toes become obstructed by atheromatous embolization causing toe ischemia (Fig. 47-1). Livedo reticularis (localized mottling of the skin) occurs when the atheroembolism involves small cutaneous vessels (Figs. 47-2 and 47-3). When present, this can be an important diagnostic indicator of atheroembolism. Acute and chronic kidney failure can result from aortic or renal artery atheroembolism. Atheroemboli can also travel to the mesenteric arteries, causing intestinal necrosis, or to the splenic, hepatic, or pancreatic arteries, causing localized infarction. Transient ischemic episodes and stroke may result from atheromatous disease of the aortic arch, internal carotid, or vertebral arteries. Atheroembolism to the retinal arteries may present with temporary horizontal monocular visual loss called amaurosis fugax. Funduscopic examination may identify a bright reflection from a cholesterol crystal in a retinal artery known as a Hollenhorst plaque.3 The unifying cause of all atheroembolic syndromes is the presence of atheromatous disease in a proximal artery and ischemic damage to a distal organ or extremity when these fragments embolize and lodge in distal vessels.
![]() Box 47-1 Clinical Syndromes and Manifestations of Atheroembolism
Box 47-1 Clinical Syndromes and Manifestations of Atheroembolism
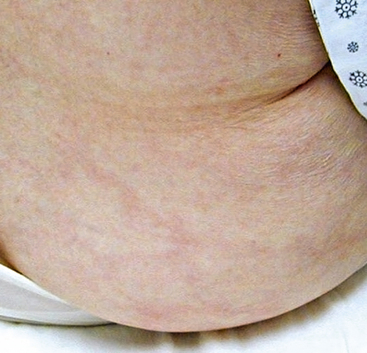
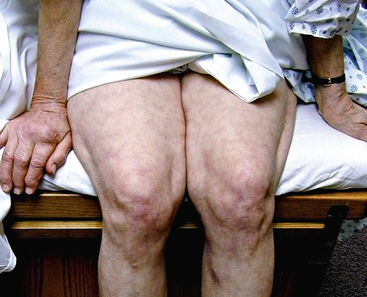
Figure 47-3 Atheroembolism to buttock and flank. Same patient as in Figure 47-2, with renal failure and livedo reticularis as presenting symptoms of previously unknown abdominal aortic aneurysm (AAA).
A number of terms for this syndrome are used interchangeably in the literature, including cholesterol crystal embolization, atheromatous embolization, and atheroembolism. Vascular medicine covers a great deal of internal medicine, and atheroembolism should be in the differential diagnosis of many diseases including vasculitis, infective endocarditis, malignancy, hematological diseases, atypical infections, Raynaud’s syndrome, and acute and chronic renal failure. Atheroembolism has been called “the great masquerader” because it may resemble many other conditions.4 Diagnosis of atheroembolism is usually made on the basis of clinical suspicion, by history and examination, but most importantly by an astute clinician who considers this entity when presented with unusual vascular diseases.
Pathobiology
Atheroembolism originates from atherosclerotic plaque. The pathobiology of atherosclerosis is reviewed in detail in Chapter 8.
Etiology
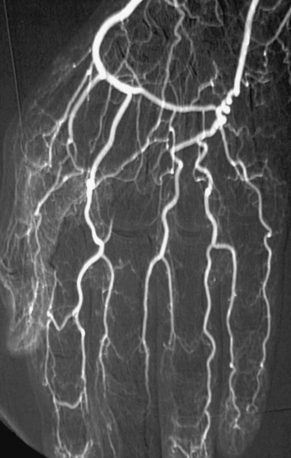
Atheroembolism may occur spontaneously or be precipitated by angiographic or surgical procedures (iatrogenic). Earlier reports indicated spontaneous episodes of atheroembolism were more common.5,6 Today with increased numbers of vascular procedures, iatrogenic catheter-induced atheroembolism outnumbers spontaneous cases. Currently, over three fourths of atheroembolic renal disease is procedure-related, occurring during or after an angiographic or endovascular procedure7,8 (Figs. 47-4 through 47-6).
Spontaneous Atheroembolism
Who is at risk?
Spontaneous atheroembolism occurs in older patients with advanced atherosclerosis. In Fine’s review of 221 cases of atheroembolism in the English literature, he noted a predominance of patients with underlying atherosclerotic disease including cardiac, carotid, and kidney disease. In particular, many had significant preexisting chronic kidney disease (CKD) evidenced by elevated serum creatinine (Cr) (CKD stage 3 or 4). There was also a high incidence of aortic aneurysms, present in 25% of these patients.5 Many patients with atheroembolic events present with multisystem manifestations. Common presentations of spontaneous atheroembolism included blue toe syndrome, livedo, and progressive renal failure. Stroke/transient ischemic events due to carotid atherosclerosis is one of the best examples of a spontaneous atheroembolic episode. The mechanism of blue toe syndrome has been likened to a brain transient ischemic attack (TIA), but affecting a lower extremity.9 Today, many cases of unexplained progressive renal failure may be due to unsuspected atheroembolism.
In most series, males outnumber females, with mean ages ranging from 63 to 69 years.1,5,10 Skin lesions and renal failure are often the two most common manifestations.1,5 Skin lesions may be the initial clinical sign of atheroembolization (see Fig. 47-3), of which blue toes and livedo reticularis make up 88% of cutaneous findings.1 African Americans are less likely to be diagnosed with atheroembolism, perhaps because the faint cutaneous pattern of livedo is more difficult to see in skin that is more deeply pigmented11 (Fig. 47-7).
How common is spontaneous atheroembolism?
Autopsies studies from the 1940s found an atheroembolic incidence of 3.4% (9 of 267 patients with aortic atherosclerosis).12 A larger and more recent autopsy study involving 2126 elderly patients over a period of 7 years found only 16 cases of spontaneous atheroembolism (incidence <0.1%) despite the high prevalence of severe aortoiliac atheromatous disease and aortic aneurysms.13 Another autopsy study of 372 patients found spontaneous cholesterol embolization occurred in only seven individuals; all were over age 60, and all but one were male, for an incidence of 1.9%. Lesions of different ages were noted, suggesting recurrent episodes to the kidneys and spleen.10 A review of autopsy data at Johns Hopkins from 1973 to 1995 found 0.7% had histological features of atheroembolism.14
The actual incidence of spontaneous atheroembolism is difficult to determine; symptoms may be vague, and clinical features can be subtle or not recognized.15
Procedure-Related Atheroembolism
Cardiovascular surgery
Atheroembolism can occur as a complication of any invasive cardiac or vascular procedure or operation. Atheroembolism has been reported following abdominal aortic aneurysm (AAA) repair, aortoiliac bypass, and aortic and renal arteriography. Atheromatous debris can be dislodged during left heart catheterization, external cardiac message, blunt abdominal trauma, coronary artery bypass surgery, and many endovascular procedures.2,16
The advent of aortography in the 1930s transformed our ability to make accurate vascular diagnosis and expanded treatment options, but brought with it the risk of atheroembolism, especially in patients with atheromatous or aneurysmal disease. A 30% incidence of atheroembolism after abdominal aortography was reported in patients who subsequently died within 6 months of the procedure. The organs most commonly affected were the kidneys and spleen.17
In the 1950s, Thurlbeck and Castleman first reported atheroembolism associated with vascular surgery and attributed this to operative manipulations that included arterial incisions, cannulation, and clamping of major arteries. Postmortem examination of those who died following AAA repair found cholesterol embolization to the kidneys in as many as 77% of patients.18 In a more recent large retrospective series of 1011 patients undergoing angiographic procedures prior to aortic or infrainguinal vascular surgery, 2.9% (29 patients) were identified with atheroembolism. The majority of iatrogenic cases were attributed to aortography as opposed to surgery. The primary sources of embolism in these patients were the abdominal aorta, iliac arteries, and femoropopliteal arteries.19
Massive atheroembolism following aortoiliac stent placement for treatment of aortic aneurysmal disease has been reported but is uncommon.20,21 A study using Doppler ultrasound to identify microembolism found a significantly higher degree of peripheral embolization during endovascular aneurysm repair, compared to conventional surgical aneurysm repair.22
Atheroembolism can also occur after coronary artery bypass grafting (CABG) and valve surgery. Fatal myocardial infarction (MI) due to atheroembolism has been reported during coronary artery bypass operations.23 Procedures that provoke atheroembolism include aortic cannulation, initiation of cardiopulmonary bypass (CPB), and transecting and anastomosis of bypass grafts to the ascending aorta during cardiac surgery. Atheroemboli may originate from the aortic root at the origin of vein grafts or from ruptured plaque in a coronary artery. In one series of 29 patients who died after cardiac surgery, atheroembolism was the causative factor in 22% of all deaths. In this series, atheroembolism to the coronary circulation caused cardiac failure; to the brain caused massive stroke; and to the gastrointestinal tract caused abdominal pain and bleeding.14 Fortunately this is rare. Of 4095 CABG procedures, atheroembolism was found in only nine patients, for an incidence of 0.22%.23 Those undergoing reoperation were found to have a higher incidence of 2.3%.23
Atherosclerosis involving the ascending aorta is a major risk factor for stroke during cardiac surgery. A large autopsy study of 221 patients found severe atherosclerosis of the ascending aorta in patients who died from atheroembolism after cardiac procedures. Atheroembolism occurred in 46 of 123 patients with severe ascending aortic disease, but only 2 of 98 (2%) without ascending aortic disease.24 The incidence of atheroembolism was three times as high after CABG than valve surgery (26.1% vs. 8.9%).24 Older patients with more advanced atheromatous disease of the ascending aorta were at the highest risk. Atheroemboli traveled to the brain in 16%, the spleen in 11%, kidney in 10%, and pancreas in 7%. Two thirds of patients had multiple sites of atheroembolism.24 A 12% stroke risk during CPB was noted in a more recent study if aortic arch atheromas are seen with intraoperative transesophageal echocardiography (TEE).25
Transcranial Doppler (TCD) has documented that the greatest number of microembolic events was found during aortic clamping and initiation of bypass.26 A study where an intraaortic filter was deployed and left in place until the patient was weaned from bypass found that 62% of filters contained atheroma in addition to platelet and fibrin strands.27
Off-pump CABG may reduce cerebral damage due to microembolism.28 A study comparing effects of CABG with and without CPB assessed retinal microembolization by fluorescein angiography and fundus photography. Doppler high-intensity transient signals (HITS) was used to assess emboli to the brain. Five of nine pump patients had retinal microvascular damage, but none was evident in the off-pump patients. Doppler HITS were 20 times more frequent in the CPB patients.28 Off-pump coronary artery bypass may have less risk of atheroembolism by avoiding arterial cannulation and the abrasive effect of CPB on the arterial wall.24,28
Cardiac catheterization
Cholesterol embolization after left heart catheterization is rare but can be devastating when it occurs. Coronary angiography is the most common invasive procedure associated with atheroembolism.8 Clinically detectable cholesterol embolization occurring after cardiac catheterization has resulted in stroke, renal failure, mesenteric ischemia, and lower-extremity tissue loss with gangrene. In some situations it has a high fatality rate.29–33
A retrospective British study by Drost et al. reported 7 cases out of a total of 4587 cardiac catheterization procedures. Most had extensive atherosclerosis and suffered multisystem atheroembolization, with a retinal embolism in one patient, renal failure in five patients, and three requiring toe amputations. Six of the seven had extensive atherosclerosis. Four of these patients died within 4.5 months of this procedure.29
In a large prospective Japanese study, Fukumoto et al. prospectively reviewed 1786 consecutive patients undergoing left heart catheterization in a multicenter study. Diagnostic criteria for atheroembolism included livedo reticularis, blue toe syndrome, digital gangrene, or renal dysfunction. They found an incidence of 1.4 % (25 patients), of which cutaneous findings and renal failure were the two most common clinical findings. If only definite cases were counted, the incidence was lower at 0.75%. Prognosis is poor in some patients, with an in-hospital mortality rate of 16 %.34
Saklayen et al. prospectively evaluated 267 patients undergoing coronary angiography at a Veterans Administration (VA) medical center. A rise in Cr of 0.5 mg/dL or more at 3 weeks after the procedure was the main indicator of atheroembolism. They found an incidence of atheroembolic renal dysfunction of 1.9% (5/263 of patients undergoing coronary angiography).31 Frock identified 17 patients with atheroembolic renal disease out of 14,998 angiographic procedures, an incidence of 0.1%.35 In a prospective study of 1579 patients, Johnson et al. also found the incidence of cholesterol embolization to be very low: a single patient in 1579 coronary angiograms (0.06%).36
Passage of a catheter into the aorta for any endovascular procedure may not only loosen atheromatous plaque but can also scrape off aortic debris into the coronary guiding catheters. During contrast injection, this debris could be injected into the coronary or cerebral artery. In a study of 1000 consecutive coronary interventions, the amount of atheromatous material entering a guiding catheter from passage up the aorta was assessed by allowing blood to passively exit the back of the catheter into a sterile towel. Depending on the catheter shape, aortic debris was recovered in 24% to 65% of interventional cases. Surprisingly, the finding of aortic debris did not correlate with clinically apparent neurological, coronary, or renal ischemic events. Allowing adequate back-bleeding of guiding catheters before injecting contrast was suggested to decrease the risk of atheroembolism found in the guiding catheter from scraping the wall of the aorta during placement.37
Today with more advanced surgical techniques and greater awareness of atheroembolic risk, the incidence of atheroembolism during vascular and cardiac procedures is less common. The promise of distal protection devices to decrease the risk of atheroembolism during a procedure is still being assessed. Atheromatous debris can be recovered from the majority of angioplasty procedures. Development of more flexible catheters and lower-profile devices, along with improved operator technique, should allow for lower incidence of atheroembolic events in the future.38
Intraaortic Balloon Pumps
Karalis et al. addressed the risk of atheroembolism in patients undergoing catheterization when they have a so-called shaggy aorta. In this study, 70 patients were identified with aortic debris found on echocardiography, and 10 had a procedure-related embolic episode. A brachial approach may be a better option in these patients.39
Intraaortic balloon pumps have especially high potential for embolization when placed in an aorta with atherosclerotic debris. In one study, 5 of 10 patients (50%) had an embolic event related to placement of an intraaortic balloon pump.39 If aortic debris is mobile, risk of embolization is especially high.
Spinal cord atheroembolism is a very rare complication of angiography.13,40,41 Spinal cord infarction likely occurs secondary to embolic occlusion of small spinal cord arteries.
Anticoagulation/Thrombolysis Issues
The concern that warfarin could precipitate atheroembolism was first raised in 1961 by Fedar and Auerbach, who reported six patients who had developed painful purple toes after initiation of an oral anticoagulant.42 Since then, anecdotal reports have linked warfarin with spontaneous atheroembolism. Clinical improvement of atheroembolism manifestations, including livedo reticularis, abdominal pain, and even renal function, has been reported after oral anticoagulants were discontinued.43,44 Other anecdotal reports have indicated improvement in skin necrosis and livedo when low-molecular-weight heparin (LMWH) was discontinued.43,45 It is hypothesized that anticoagulation could dissolve or prevent formation of a protective thrombus over an atherosclerotic plaque, leaving it vulnerable to rupture and embolization.
To the contrary, a number of large studies have shown no increased risk of atheroembolism in patients treated with warfarin. Blackshear et al. addressed concerns of warfarin anticoagulation in patients with atrial fibrillation and documented aortic plaque. The SPAF III (Stroke Prevention in Atrial Fibrillation) trial looked at patients with atrial fibrillation and aortic plaque documented by TEE and found that patients assigned to warfarin therapy had an annual cholesterol embolization rate of 0.7 per patient-year, which was lower than those randomized to warfarin plus aspirin. The authors conclude that “elderly patients with AF and aortic plaque can receive adjusted-dose warfarin with a relatively low risk of cholesterol embolism.”46 The French Study of Aortic Plaques in Stroke found no difference in recurrent brain infarction in patients receiving warfarin compared to those on aspirin.47
Many cardiovascular patients are on anticoagulation, and in most case reports, causation is difficult to prove. Elderly patients are most likely to have advanced atherosclerosis and yet have chronic disorders such as atrial fibrillation requiring long-term anticoagulation. Often these patients had undergone other procedures including angiography, which is more likely an explanation for atheroembolic events. Delayed recognition of an acute event or recurrent showers in patients with shaggy aortas may account for the temporal association of atheroembolism with an oral anticoagulant.48
A sensible conclusion is to continue anticoagulation when compelling conditions exist, such as atrial fibrillation and thromboembolism, but consider stopping it if there is a lesser indication.38
Atheroembolism has also been reported to occur after thrombolytic therapy for myocardial infarction.49–52 In some of these case reports, atheroembolism occurred in the absence of any invasive procedure, therefore implicating thrombolysis as a possible culprit. The mechanism of atheroembolism is thought to be dissolution of thrombus overlying atheromatous plaque, exposing ulcerated plaque to the arterial circulation that can embolize distally. Large trials of thrombolysis for acute myocardial infarction (AMI), including GISSI 2 and GUSTO, did not cite atheroembolism as a complication of thrombolytic therapy, so concern of atheroembolization should not be a reason to withhold thrombolysis.53
Blankenship et al. prospectively followed 60 patients with AMI who later underwent CABG. Half of these patients received thrombolytic therapy and half did not. It was concluded the prevalence of cholesterol embolization was not higher in those who received thrombolytic therapy.54
Atheroembolic Syndromes
Livedo and Skin Atheroembolism
Skin findings are the earliest and at times the only clinical finding of atheroembolism. Livedo reticularis is the most common manifestation of skin involvement, noted in 49% of patients.55,56 The incidence of cutaneous manifestations of atheroembolism ranges from 35% to 96%.1,57,58 Livedo has been labeled an underutilized clue to the diagnosis of atheroembolism because it should be considered an important and common indicator of atheroembolism elsewhere—in particular, to the kidneys or mesenteric organs.55,59 For example, the suspicion of renal atheroembolism is markedly strengthened by the finding of livedo reticularis affecting the trunk or abdomen.
Livedo reticularis has a classic appearance as a reddish-blue lacy or netlike color pattern of the skin (Fig. 47-8). It is noted when the cutaneous venous plexus becomes visible owing to desaturated venous blood. In the presence of atheroembolism, small arteries are obstructed, reducing flow into the venous plexus and resulting in stasis of deoxygenated blood.60 Characteristics of livedo reticularis include blanching with local pressure. It is more prominent when the patient is upright and may not be apparent if the patient is examined in the supine position.55 Atheroembolism is less frequently diagnosed in nonwhite individuals because darker skin may disguise visible manifestations.11 Livedo is most commonly seen on the feet and legs, but thighs, buttocks, lower back, abdomen, and upper-extremities can also be affected, depending on the source of the atheroembolism.1
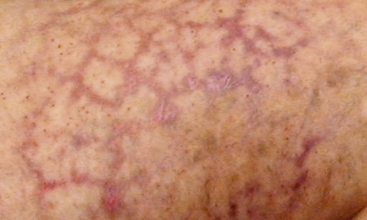
Figure 47-8 Unusual nonblanching livedo reticularis of thigh in patient with suspected livedo vasculitis.
Less common cutaneous findings in atheroembolism include splinter hemorrhages, petechiae, purpura, and erythematous nodules. Cholesterol embolism to the skin has been called a pseudovasculitic syndrome.61 Livedo due to atheroembolism has been mistaken for small-vessel vasculitis in 16% of patients.62 The diagnosis of atheroembolism can be confirmed by skin biopsy, and is positive in 92% of cases.57,62
In young women, livedo reticularis may be a common normal finding due to cold-induced vasospasm of skin vessels, and classically disappears with warming. It can also be seen in a number of diseases including collagen vascular disorders, antiphospholipid antibody syndrome, disseminated intravascular coagulation (DIC), vasculitides such as systemic lupus erythematosus (SLE) and polyarteritis nodosa, infective endocarditis, cryoglobulinemia, and hyperviscosity disorders.38
Atheroembolic Renal Disease
About half of all reported cases of atheroembolism involve the kidney.7 The kidney receives a major percentage of the cardiac output and is the most common site for atheroembolism, followed by skin and gastrointestinal tract.1,63 In clinical practice, it has been estimated that up to 10% of all cases of acute renal failure may be due to atheroembolism.64
Atheroembolic renal disease is defined as a syndrome of renal failure secondary to obstruction of small kidney arteries, arterioles, and glomerular capillaries by cholesterol crystal atheroembolism dislodged from the aorta or proximal renal arteries.65 Renal atheroembolism may occur spontaneously in patients who have advanced atheromatous disease of the abdominal aorta, but more frequently it occurs as a complication of an angiographic or endovascular procedure. As noted earlier, passage of a catheter or guidewire through the aorta or renal arteries may dislodge atheromatous plaque fragments that travel to the kidneys, where they remain in small vessels. Today, approximately three fourths of renal atheroembolization cases are iatrogenic secondary to invasive procedures, in particular angiography. Coronary angiography is the most common angiographic procedure.7,8
The exact incidence of spontaneous atheroembolic renal disease is difficult to determine because most studies are retrospective. However, approximately 20% of atheroembolic episodes to the kidneys are thought to be unprovoked spontaneous episodes.65 In addition, many atheroembolic episodes are subclinical, difficult to diagnose, and may be missed unless specifically looked for.
In renal biopsy studies, the prevalence of renal atheroembolism in all patients and age groups is quite low, ranging from 0.31% to 2.4%.8,10 Moolenaar and Lamers reviewed the Netherlands experience of 842 cases of cholesterol crystal embolization in the Dutch National Pathology Information System and found an incidence of 6.0 cases per million population. Among autopsy reports, they also found a low incidence of 0.31%.63 In other renal biopsy series, Greenberg et al. found 24/500 had atheroembolic findings.66 In another large series of 755 renal biopsies, atheroemboli were discovered in 8 patients (1.1%).67 Selection bias may account for the low prevalence; these patients were selected for biopsy because of unexplained recent worsening of renal function.
Atheroembolism to the kidneys can also occur during any vascular surgical procedure, in particular AAA resection, renal revascularization, and aortoiliac or aortofemoral bypass. In those who died after aortic surgery or an angiographic procedure, the finding of atheroembolism at autopsy ranged from 12% to 77%.8 Atheromatous emboli to the kidney was documented in 77% of patients following surgical repair of abdominal aortic aneurysms.2
Atheroembolism to the kidneys also occurs as complication of endovascular procedures (Box 47-2). Modi and Rao reports 85% of patients presenting with atheroembolic renal disease underwent an invasive vascular procedure within the prior 3 months (abdominal aorta, coronary, or carotid angiography).65 Olin has stated that atheroembolism likely occurs in every patient undergoing an endovascular procedure (renal artery angioplasty and stent) for atherosclerotic renal disease.68 A study by Kawarada et al. used intrarenal duplex ultrasound to detect microembolic signals and found that emboli occurred in all 13 patients undergoing renal artery stent implantation, in particular post dilation of the stent.69 Underappreciation of this frequent occurrence is because clinicians attribute acute renal failure after a procedure to another cause such as contrast-induced nephropathy.
A prospective study at a VA medical center looked at renal failure after cardiac catheterization. Atheroembolism was suspected on the basis of a 0.5 mg/dL or more rise in Cr 3 weeks after a coronary angiogram. Although the incidence of renal impairment was low in this group, two of the five died of renal failure. Of note, none of the five had skin signs of livedo, and the diagnosis of atheroembolism would have been missed on examination.31
Atheroembolism to the kidneys is underrecognized as a cause of acute and chronic renal disease. In one review of 259 patients who underwent renal biopsy for acute renal failure, cholesterol emboli were found in 6.9% of cases. Of note, 15 of 18 of these patients were clinically unsuspected to have atheroembolism as a cause of renal failure. Another study found 7.5% of patients with acute renal failure had documented atheroembolism on renal biopsy.70
In one review, Mayo and Swartz estimated that 4% of all inpatient nephrology consults were due to atheroembolism.64 This may be a conservative estimate because older patients with multiple risk factors accounted for a higher proportion of in-hospital nephrology consults. Of those consults seen with acute renal failure, 5% to 10% were felt to be due to atheroembolic renal disease.64
Atheromatous emboli and cholesterol crystals tend to lodge in arcuate and interlobar arteries which are 150 to 200 microns in diameter.8,65 Cholesterol crystal emboli not only cause mechanical vessel obstruction but also set up an endothelial inflammatory reaction that some have labeled microcrystalline angiitis.8 This is characterized by polymorphonuclear leukocyte and eosinophil infiltration around the vessel, and subsequently mononuclear cells with giant cell formation in the perivascular tissues. Endothelial distortion, intimal proliferation, perivascular fibrosis, and sometimes extraluminalization of crystals can be seen.67 With thrombus formation, there is further microvascular occlusion of renal vessels. Over 2 to 4 weeks, there is a progressive gradual decline in renal function following an acute atheroembolic episode. Renal infarction or necrosis is rare because the process is patchy and does not obstruct the larger feeding arteries to the kidney.
Atheroembolic renal disease presents as acute or subacute renal dysfunction in older patients, rarely younger than age 50, usually affecting those with preexisting renal insufficiency.5,35,65,71 Patients with atheroembolism have multiple risk factors including smoking, diabetes, hypertension, and hyperlipidemia. A review of 52 cases of atheroembolic renal failure at the Massachusetts General Hospital from 1981 to 1990 found these patients were more likely to have significant hypertension and coronary and peripheral artery disease (PAD).72
The clinical course may be variable. In contrast-induced nephropathy, renal failure occurs immediately after dye infusion, with a peak in Cr within several days and resolving in less than 2 weeks.8 Unlike contrast-induced nephropathy, atheroembolic renal failure may slowly worsen over a period of weeks to months, likely because of recurrent spontaneous showers of emboli. The kidney responds to ischemic damage with inflammatory changes that lead to glomerular sclerosis, tubular atrophy, and interstitial fibrosis.38,65 Sometimes, features of focal segmental necrotizing glomerulonephritis and crescentic glomerulonephritis are seen in renal biopsy specimens.65,66,73
Atheroembolism to kidneys may be subclinical. Subacute presentation is more common with progressive renal failure over several weeks. In one report, the average time interval between an angiographic procedure and diagnosis of atheroembolic renal disease was 5 weeks.35 Renal function declined over 3 to 8 weeks.72
A chronic form of renal failure may be mild and can be clinically silent. Atheroembolism may present acutely, with onset within 1 week, or be subacute with delayed onset of renal impairment 2 to 6 weeks after an inciting procedure. A step-and-plateau drop in renal function has been described, perhaps owing to intermittent recurrent showers of cholesterol crystals over a period of time. One to two thirds of patients with atheroembolic renal disease will need dialysis. Lye et al. reviewed the English literature in 1993, noting that 40% of patients required dialysis.6 Only 20% to 30% will recover sufficient renal function to stop dialysis.6,8,74
Clinical features are often absent but may include flank pain and gross hematuria due to renal infarction. Abdominal pain, nausea, vomiting, and blood loss can result from embolization to the gastrointestinal tract. In approximately half of these patients, there may be livedo reticularis or purple toe discoloration due to cholesterol embolization to the skin. Fever, malaise, and weight loss may be accompanying systemic symptoms.65
Severe or resistant hypertension has been noted in up to half of patients with atheroembolic renal disease.6,15 Hypertension may result from activation of renin angiotensin system, or be volume mediated secondary to inability of the kidney to excrete excess fluid. Malignant hypertension can occur with atheromatous embolization to the kidneys.75
Laboratory testing
Laboratory testing generally shows nonspecific findings. Although elevated creatinine, proteinuria, and eosinophilia have been reported in up to 80% of patients in the acute stage, these findings are inconsistently found.15,76 Anemia, leukocytosis, thrombocytopenia, and elevated inflammatory markers including sedimentation rate and C-reactive protein (CRP) are occasionally noted.1
The finding of eosinophils in the urine has been considered to be a very important diagnostic feature of renal atheroembolism. In a report by Wilson et al., urine eosinophils were found in 8 of 24 patients who had biopsy-proven atheroembolic disease. Six of the eight patients had more than 5% of urinary white cells as eosinophils.77 Hansel’s stain may increase the identification of urinary eosinophils. Urinary eosinophils, however, can be seen in other kidney disorders such as acute interstitial nephritis and other allergic disorders. Urinalysis may show hyaline or granular casts. Proteinuria may be present but is rarely severe enough to cause nephrotic syndrome.78 Urine sediment is usually inactive and unremarkable.78,79
Blood tests may show eosinophilia ranging from 14% to 80%, but again this is not a consistent finding.8,72 Eosinophilia is thought to be due to inflammatory changes in the kidney with immune activation. Kasinath et al. reviewed the literature and observed that 29 of 36 reports noted eosinophilia in association with renal atheroembolism. In this patient series, they found eosinophil counts ranging from 540 to 2000 cells/mm3.76 Modi and Rao found that 60% of patients had eosinophilia,65 and Lye et al. reported an incidence of 71%.6 Eosinophilia may be transient and seen only in the acute phase. Despite not always being present, if the eosinophil count is greater than 500 cells per μL, many clinicians feel this is a contributing finding, helpful in establishing a possible diagnosis of atheroembolism.7
The definitive diagnosis of atheroembolic kidney disease is confirmed histologically by the demonstration of cholesterol crystals in arcuate and interlobular arteries of the kidney. The sensitivity of a single renal biopsy may be only 75% owing to the patchy distribution of atheroembolism; however, with two biopsies, 94% are positive.65
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree