Metabolic syndrome (MS) and atrial fibrillation (AF) are associated with increased cardiovascular disease morbidity and mortality. This analysis evaluated the association between MS and AF in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. MS was defined using criteria recommended in the joint interim statement from several international societies. AF was defined by electrocardiogram (ECG) and/or self-report and by ECG alone. In patients with 0, 1, 2, 3, 4, and 5 MS components, prevalences of AF by ECG and/or self-report were 5.5%, 7.7%, 8.2%, 9.2%, 9.6%, and 11.5%, respectively (p for trend <0.001). After multivariable adjustment, each MS component except serum triglycerides was significantly associated with AF. The multivariable-adjusted odds ratio for AF, defined by ECG and/or or self-reported history, comparing those with to those without MS was 1.20 (95% confidence interval 1.10 to 1.29). Results were consistent when AF was defined by ECG alone (odds ratio 1.15, 95% confidence interval 0.92 to 1.39). In conclusion, MS is associated with an increased prevalence of AF. Further studies investigating a potential mechanism for this excess risk are warranted.
Many metabolic syndrome (MS) components (i.e., increased blood pressure, high glucose, dyslipidemia, and abdominal adiposity ) are also risk factors for atrial fibrillation (AF). However, there are limited data evaluating the association between MS and AF. Quantifying the burden of AF in patients with MS may provide justification for further studies investigating potential mechanisms of excess cardiovascular disease risk in this population. Accordingly, the goal of this analysis was to evaluate the association of MS with AF in a large population-based national study of United States adults. To do so, we analyzed data from the REasons for Geographic and Racial Differences in Stroke (REGARDS) study.
Methods
The REGARDS study is a national population-based observational study of African-American and white United States adults ≥45 years of age. Details of the study design and recruitment have been published previously. In brief, the study was designed to oversample African-Americans and residents of the geographic region referred to as the “Stroke Belt,” which consists of North and South Carolinas, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana.
The REGARDS study enrolled 30,239 participants from January 2003 through October 2007. Subjects without electrocardiographic (n = 708), blood pressure (n = 78), serum glucose (n = 1,123), serum lipid (n = 544), or waist circumference (n = 108) data were excluded from the present analysis. In addition, participants who did not answer the question about having a previous diagnosis of AF (n = 24) and those not fasting (n = 3,638) or missing fasting status (n = 105) and having poor quality electrocardiogram (ECG; n = 261) were excluded, leaving data from 23,650 participants for analysis. The REGARDS study protocol was approved by institutional review boards governing research in human subjects at the participating centers and all participants provided written consent.
Data were collected through a computer-assisted telephone interview followed by an in-home examination. Of relevance to the present analysis, the following demographic and behavioral information was collected during the interview: age, gender, race, education, annual household income, frequency of physical activity, smoking status, nonsteroidal anti-inflammatory drug use, previous stroke, and current use of antihypertensive and antidiabetic medications. The in-home examination included clinical measurements, an ECG, and collection of a fasting blood sample and urine sample. Left ventricular hypertrophy was defined from Cornell voltage criteria as described previously. Clinical data (height, weight, waist circumference, blood pressure) were collected according to standardized protocols. Two blood pressure measurements were taken and averaged for analysis. Using a spot urine, the albumin-to-creatinine ratio was calculated and categorized as no albuminuria (<30 mg/g), microalbuminuria (30 to 299 mg/g), or macroalbuminuria (≥300 mg/g). Estimated glomerular filtration rate was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. C-reactive protein (CRP) was measured using a high-sensitivity particle-enhanced immunonephelometric assay, with levels ≥3 mg/L defined as increased.
MS was defined using criteria recommended in the joint interim statement of the International Diabetes Foundation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Specifically, increased blood pressure was defined by systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or current antihypertensive medication use; low high-density lipoprotein (HDL) cholesterol was defined as <40 mg/dl in men and <50 mg/dl in women; high serum triglycerides was defined as ≥150 mg/dl; high fasting plasma glucose was defined as ≥100 mg/dl or antidiabetic medication use; and abdominal obesity was defined as a waist circumference >102 cm in men and >88 cm in women. MS was defined as the presence of ≥3 of the 5 components. In a secondary analysis, CRP level was included as a sixth component, with CRP-MS defined as ≥3 of these 6 components.
During the initial stages of the REGARDS study, AF was detected from a 7-lead electrocardiographic recording using a single midsternal chest lead (n = 6,507). However, a standard 12-lead ECG was used later in the study (n = 17,143). Because ECG-diagnosed AF is not affected by the number of leads, participants with 7- and 12-lead ECGs were included in this analysis. All ECGs were read and coded at a central reading center by trained personnel masked to clinical data collected in the REGARDS study. For the primary analysis, AF was defined by self-report and/or ECG. This method has been shown to be more sensitive to detection of AF than standard 12-lead ECGs alone. Further, self-report is a common method for AF ascertainment in epidemiologic studies, and associations of morbidity and mortality with self-reported AF are similar to those with ECG-detected AF. A self-reported history of AF was defined as answering “yes” to the following question: “Has a physician or a health professional ever told you that you had atrial fibrillation?” Analyses were also conducted using AF determined by ECG alone as the outcome.
The percentage of REGARDS study participants having 0, 1, 2, 3, 4, or 5 MS components was calculated. Characteristics of the study participants with and without MS were determined. Statistical significance of differences in variables across MS status was determined using t tests, chi-square tests, or Mann–Whitney tests, as appropriate. Prevalence of AF was calculated for study participants with and without each MS component and according to number of MS components. Odds ratios (ORs) for AF associated with each MS component were calculated using logistic regression. These were calculated unadjusted and after adjustment for age, gender, race, education, income, physical inactivity, smoking, nonsteroidal anti-inflammatory drug use, estimated glomerular filtration rate, microalbuminuria, macroalbuminuria, and left ventricular hypertrophy. Because of the strong association between stroke and AF, a sensitivity analysis was conducted by repeating the main analyses after excluding REGARDS study participants with a history of stroke. We performed secondary analyses using CRP level as a sixth MS component (CRP-MS). First, prevalence of AF was calculated for participants with and without increased CRP. Then, we calculated prevalence of AF in those with or without CRP-MS. In addition, the OR for AF associated with increased CRP and for CRP-MS was calculated after adjustment as described earlier. For an additional secondary analysis, multivariable-adjusted ORs for AF associated with each component, number of components, and MS were calculated for whites and African-Americans separately. Effect modification by race was assessed by including multiplicative interaction terms in the regression model (e.g., race by MS). All analyses were conducted using SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
Overall, 39.8% of study participants had MS. Prevalences of 0, 1, 2, 3, 4, and 5 MS components were 12.4%, 22.5%, 25.2%, 21.7%, 13.4%, and 4.8%, respectively. Characteristics of the study population by MS status are presented in Table 1 .
| Variable | MS | p value | |
|---|---|---|---|
| Yes | No | ||
| (n = 9,421) | (n = 14,229) | ||
| Age (years) | 64.7 ± 9.0 | 64.5 ± 9.7 | <0.001 |
| Women | 58.4% | 53.1% | <0.001 |
| African-American | 44.6% | 36.8% | <0.001 |
| High school education | 85.5% | 90.4% | <0.001 |
| Income <$20,000 | 24.4% | 15.8% | <0.001 |
| Height (inches) | 66.7 ± 4.4 | 66.8 ± 4.0 | <0.001 |
| Weight (kg) | 94.0 ± 19.8 | 77.8 ± 16.2 | <0.001 |
| Body mass index (kg/m 2 ) | 32.6 ± 6.1 | 27.0 ± 5.0 | <0.001 |
| Physically inactive | 40.0% | 29.5% | <0.001 |
| Currently smoke | 14.9% | 14.0% | 0.07 |
| Nonsteroidal anti-inflammatory drug use in previous month | 16.6% | 13.2% | <0.001 |
| Albumin-to-creatinine ratio (mg/g) | 8.7 (5.1–22.0) | 6.5 (4.3–12.2) | <0.001 |
| Microalbuminuria ⁎ | 15.8% | 8.7% | <0.001 |
| Macroalbuminuria † | 4.2% | 1.5% | <0.001 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 83.6 ± 22.0 | 86.8 ± 18.2 | <0.001 |
| C-reactive protein (mg/L) | 3.2 (1.5–6.8) | 1.6 (0.8–3.8) | <0.001 |
| Systolic blood pressure (mm Hg) | 132.1 ± 16.5 | 124.2 ± 16.0 | <0.001 |
| Diastolic blood pressure (mm Hg) | 78.3 ± 9.9 | 75.5 ± 9.7 | <0.001 |
| Currently using antihypertensive medication | 72.9% | 38.6% | <0.001 |
| Plasma glucose (mg/dl) | 114.9 ± 38.8 | 93.0 ± 20.0 | <0.001 |
| Serum high-density lipoprotein cholesterol (mg/dl) | 44.5 ± 12.7 | 57.4 ± 16.0 | <0.001 |
| Serum low-density lipoprotein cholesterol (mg/dl) | 113.4 ± 36.3 | 116.0 ± 33.5 | <0.001 |
| Currently using lipid-lowering medication | 40.6% | 28.2% | <0.001 |
| Serum triglycerides (mg/dl) | 150.0 (103.0–199.0) | 94.0 (72.0–122.0) | <0.001 |
| Waist circumference (cm) | 104.9 ± 14.0 | 89.7 ± 13.2 | <0.001 |
⁎ Albumin-to-creatinine ratio of 30 to 300 mg/g.
Prevalence of AF defined by ECG and/or self-report was 8.3%. Prevalence of AF was higher in participants with versus without each MS component ( Table 2 ). AF was also more common in those with a larger number of MS components present (p for trend <0.001; Figure 1 ). Overall, prevalences of AF by ECG and/or self-report were 9.6% and 7.5% for those with and those without MS, respectively (p <0.001).
| AF by ECG and/or Self-Report | ||
|---|---|---|
| n (prevalence) | p Value | |
| Systolic/diastolic blood pressure ≥130/85 mm Hg or use of antihypertensive medication | <0.001 | |
| Yes | 15,904 (9.2%) | |
| No | 7,746 (6.6%) | |
| Serum high-density lipoprotein cholesterol <40 mg/dl in men and <50 mg/dl in women | <0.001 | |
| Yes | 8,292 (9.4%) | |
| No | 15,358 (7.7%) | |
| Serum triglycerides ≥150 mg/dl | 0.03 | |
| Yes | 6,192 (9.0%) | |
| No | 17,458 (8.1%) | |
| Serum glucose ≥100 mg/dl or use of glucose-lowering medication | <0.001 | |
| Yes | 9,172 (9.4%) | |
| No | 14,478 (7.7%) | |
| Waist circumference ≥102 cm in men and ≥88 cm in women | 0.001 | |
| Yes | 11,381 (8.9%) | |
| No | 12,269 (7.8%) | |
| Metabolic syndrome | <0.001 | |
| Yes | 9,421 (9.6%) | |
| No | 14,229 (7.5%) | |
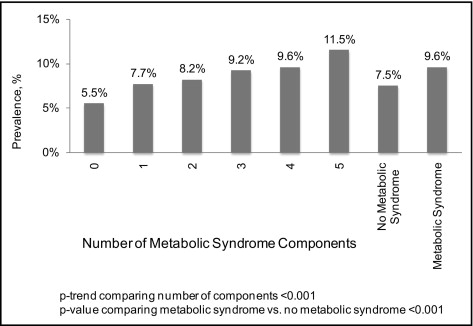
Each MS component was associated with an increased OR for AF by ECG and/or self-report in crude analyses ( Table 3 ). After multivariable adjustment, these associations were attenuated but remained statistically significant for each component except high triglycerides. As the number of MS components increased, the OR for AF increased in crude analyses and after multivariable adjustment (each p for trend <0.001). Crude and multivariable-adjusted ORs for AF comparing those with to those without MS were 1.32 (95% confidence interval [CI] 1.20 to 1.45) and 1.20 (95% CI 1.10 to 1.29), respectively. In a sensitivity analysis excluding REGARDS study participants with prevalent stroke, the multivariable-adjusted OR for AF was 1.23 (95% CI 1.13 to 1.33).
| Variable | OR (95% CI) for AF by ECG and/or Self-Report | ||
|---|---|---|---|
| Crude | Multivariable Adjusted ⁎ | Multivariable Adjusted † | |
| Systolic/diastolic blood pressure ≥130/85 mm Hg ‡ | 1.43 (1.29–1.59) † † | 1.30 (1.19–1.41) † † | 1.21 (1.10–1.32) † † |
| Serum high-density lipoprotein cholesterol <40 mg/dl in men and <50 mg/dl in women | 1.24 (1.13–1.36) † † | 1.22 (1.12–1.32) † † | 1.17 (1.07–1.27) ⁎⁎ |
| Serum triglycerides ≥150 mg/dl | 1.12 (1.01–1.24) # | 1.08 (0.97–1.19) | 1.04 (0.93–1.14) |
| Plasma glucose ≥100 mg/dl § | 1.25 (1.14–1.37) † † | 1.22 (1.13–1.32) † † | 1.17 (1.07–1.27) ⁎⁎ |
| Waist circumference ≥102 cm in men and ≥88 cm in women | 1.17 (1.06–1.28) ⁎⁎ | 1.17 (1.07–1.27) ⁎⁎ | 1.14 (1.04–1.23) # |
| Number of risk factors | |||
| 2 ∥ | 1.19 (1.05–1.35) ⁎⁎ | 1.15 (1.02–1.28) # | 1.12 (0.99–1.25) |
| 3 ∥ | 1.36 (1.20–1.55) † † | 1.30 (1.17–1.43) † † | 1.22 (1.09–1.36) ⁎⁎ |
| 4 ∥ | 1.42 (1.23–1.65) † † | 1.35 (1.20–1.50) † † | 1.26 (1.11–1.41) ⁎⁎ |
| 5 ∥ | 1.74 (1.42–2.13) † † | 1.60 (1.40–1.80) † † | 1.42 (1.21–1.63) † † |
| Metabolic syndrome ¶ | 1.32 (1.20–1.45) † † | 1.27 (1.18–1.36) † † | 1.20 (1.10–1.29) † † |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


