The predictive ability of the CHADS 2 index to stratify stroke risk may be mechanistically linked to severity of left atrial (LA) dysfunction. This study investigated the association between the CHADS 2 score and LA function. We performed resting transthoracic echocardiography in 970 patients with stable coronary heart disease and normal ejection fraction and calculated baseline LA functional index (LAFI) using a validated formula: (LA emptying fraction × left ventricular outflow tract velocity time integral)/LA end-systolic volume indexed to body surface area. We performed regression analyses to evaluate the association between risk scores and LAFI. Among 970 subjects, mean CHADS 2 was 1.7 ± 1.2. Mean LAFI decreased across tertiles of CHADS 2 (42.8 ± 18.1, 37.8 ± 19.1, 36.7 ± 19.4, p <0.001). After adjustment for age, sex, race, systolic blood pressure, hyperlipidemia, myocardial infarction, revascularization, body mass index, smoking, and alcohol use, high CHADS 2 remained associated with the lowest quartile of LAFI (odds ratio 2.34, p = 0.001). In multivariable analysis of component co-morbidities, heart failure, age, and creatinine clearance <60 ml/min were strongly associated with LA dysfunction. For every point increase in CHADS 2 , the LAFI decreased by 4.0%. Secondary analyses using CHA 2 DS 2 -VASc and R 2 CHADS 2 scores replicated these results. Findings were consistent when excluding patients with baseline atrial fibrillation. In conclusion, CHADS 2 , CHA 2 DS 2 -VASc, and R 2 CHADS 2 scores are associated with LA dysfunction, even in patients without baseline atrial fibrillation. These findings merit further study to determine the role of LA dysfunction in cardioembolic stroke and the value of LAFI for risk stratification.
Clinical risk stratification schemes for predicting stroke in patients with atrial fibrillation (AF) are derived from clinical databases and incorporate a series of heterogeneous risk factors. The mechanism by which these clinical characteristics are associated with increased stroke risk is not understood. Among several risk stratification indices, the CHADS 2 score is the most commonly used for stratifying stroke risk in patients with AF because it is simple to calculate, well validated, and endorsed in practice guidelines. Incorporation of renal dysfunction into the CHADS 2 score (R 2 CHADS 2 ) has recently been shown to improve discrimination and classification. More recently, CHADS 2 and other risk scores have been shown to predict stroke risk even in the absence of AF and with discrimination comparable with risk prediction in AF-only cohorts. These findings have raised the issue as to whether the association with stroke may be mediated by silent AF. The CHADS 2 index is composed of several clinical factors independently associated with both structural and electrical remodeling of the left atrium, such as older age, heart failure, and hypertension. Left atrial (LA) remodeling and dysfunction are known risk factors for the development of AF and for stroke in patients without AF. Therefore, we hypothesized that CHADS 2 , CHA 2 DS 2 -VASc, and R 2 CHADS 2 scores predict LA dysfunction.
Methods
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients with coronary heart disease (CHD). Details regarding recruitment methods and study design have been previously published. From September 2000 to December 2002, 1,024 outpatient subjects were recruited from 2 Department of Veterans Affairs medical centers (San Francisco Veterans Affairs Medical Center and the Veterans Affairs Palo Alto Health Care System), a university medical center (University of California, San Francisco), and 9 public health clinics (Community Health Network of San Francisco). Patients were eligible to participate if they had at least one of the following: a history of myocardial infarction (MI), angiographic evidence of stenosis of 50% or greater in ≥1 coronary vessels, exercise-induced ischemia by treadmill or nuclear testing, history of coronary revascularization, or diagnosis of CHD by an internist or a cardiologist. Subjects were excluded if they were unable to walk 1 block, had an MI within 6 months of study enrolment, or were planning to move away from the area within 3 years. On enrolment, patients completed a medical history interview, health questionnaire, physical examination, and exercise treadmill test with a stress echocardiogram.
Of 1,024 study subjects, we excluded 41 with missing data required for the calculation of LA functional index (LAFI), 8 with missing data for the calculation of CHADS 2 score, and 5 with missing data for the calculation of CHA 2 DS 2 -VASc score. The remaining 970 participants are the subjects of this secondary data analysis. The institutional review board approved this study, and all participants provided written and informed consent.
The primary predictors were the CHADS 2, CHA 2 DS 2 -VASc, and R 2 CHADS 2 indices. CHADS 2 score was determined by assigning 1 point each for the presence of congestive heart failure (CHF), hypertension, age ≥75, and diabetes and by assigning 2 points for history of stroke or transient ischemic attack (TIA). CHA 2 DS 2 -VASc score was determined by assigning 1 point each for the presence of CHF, hypertension, age 65 to 74 years, diabetes, and vascular disease (peripheral artery disease or MI) and by assigning 2 points for age ≥75, history of stroke, or TIA. R 2 CHADS 2 score was determined by adding 2 points for creatinine clearance <60 ml/min to the CHADS 2 score.
Age, sex, race, and medical history were determined from self-report. Height and weight were measured at baseline, and body mass index was calculated (kg/m 2 ). After 5 minutes in the supine position, systolic blood pressure (BP), diastolic BP, and heart rate were measured. Pulse pressure was calculated from resting BP. Baseline 12-lead electrocardiograms were obtained and read by 2 independent, blinded physicians. In the event of a disagreement, a third-blinded adjudicator was consulted. Hypertension was defined by self-report or systolic BP ≥160 mm Hg on baseline evaluation. Diabetes was defined by self-report, use of diabetes medications, or hemoglobin A1C value ≥7.0%. Histories of CHF, vascular disease, MI, stroke, and TIA were determined by self-report. Creatinine clearance was calculated using the Cockcroft-Gault formula.
The primary outcome was the LAFI. All subjects underwent resting transthoracic echocardiography at baseline. Echocardiograms were performed by 1 of the 2 trained technicians using a standardized protocol. Studies were performed using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, California). Images were obtained with subjects in the left lateral recumbent and supine positions. Images obtained during held inspiration in the standard 2-dimensional parasternal short-axis and apical 2- and 4-chamber views were planimetered with a computerized digitization system to determine end-diastolic and end-systolic left ventricle (LV) volumes by the biplane method of disks. End-diastolic and end-systolic LV volumes were determined from the moments of first mitral valve opening and closing. A single experienced reader blinded to clinical information interpreted all studies. The reproducibility of LAFI by this reader has been previously described with Bland-Altman analyses, which revealed no significant variation (intraobserver reproducibility: mean difference 0.0059, 95% confidence interval 0.015 to −0.012; interobserver reproducibility: mean difference 0.0017, 95% confidence interval 0.025 to −0.013).
The derivation and validation of the LAFI have been previously published. The LAFI was calculated as (LA emptying fraction × LV outflow tract-velocity time integral)/LA end-systolic volume index, where LA emptying fraction was defined as (LA end-systolic volume – LA end-diastolic volume)/LA end-systolic volume.
From the resting echocardiograms, LV mass was calculated using the truncated-ellipse method and indexed to body surface area. The LV ejection fraction (LVEF) was calculated as (LV end-diastolic volume – LV systolic volume)/LV end-diastolic volume. Diastolic dysfunction was separated into 3 categories on the basis of mitral flow ratios of peak velocities at early rapid filling and late filling at atrial contraction (E/A ratio) and systolic or diastolic dominant pulmonary venous flow: (1) impaired relaxation, defined as an E/A ratio of ≤0.75 and systolic dominant pulmonary venous flow, (2) pseudonormal, defined as an E/A of 0.75 to 1.5 and diastolic dominant pulmonary venous flow, and (3) restrictive, defined as an E/A of ≥1.5 and diastolic dominant pulmonary venous flow. Pulmonary artery systolic pressure was determined from resting echocardiograms as tricuspid regurgitation gradient + right atrial pressure. The tricuspid regurgitation jet was visualized with color flow mapping, and the tricuspid regurgitation gradient was measured with continuous-wave Doppler. We used the modified Bernoulli equation ( Δ P = 4v 2 ) to calculate gradients from velocities. Right atrial pressure was estimated from the size and respiratory variation of flow in the inferior vena cava.
Inducible ischemia was determined from exercise treadmill testing. All participants underwent testing according to a standard Bruce protocol and with continuous 12-lead electrocardiogram monitoring. Subjects underwent transthoracic echocardiography immediately before and after exercise. Inducible ischemia was defined as the presence of ≥1 wall motion abnormality at peak exercise.
Participants were divided into tertiles based on their CHADS 2, CHA 2 DS 2 -VASc, and R 2 CHADS 2 scores. Tertiles of CHADS 2 were 0 to 1, 2 to 3, and 4 to 6. Tertiles of CHA 2 DS 2 -VASc and R 2 CHADS 2 were 0 to 1, 2 to 3, and ≥4. Differences in baseline characteristics were compared using chi-square tests for categorical variables and one-way analysis of variance for continuous variables. Diastolic dysfunction was analyzed as an ordinal variable, as we have previously found differences in rates of cardiovascular outcomes in these 3 categories of diastolic dysfunction (no diastolic dysfunction, impaired relaxation, pseudonormal/restrictive). Pseudonormal and restrictive groups were combined for analysis because ≤5% of the study sample had restrictive filling. For each baseline variable, we tested for trend across risk score tertiles. Linear regression was performed to assess the association among CHADS 2, CHA 2 DS 2 -VASc, and R 2 CHADS 2 (as tertiles and per point increase) as predictors of LAFI. Logistic regression was performed to assess tertiles of CHADS 2, CHA 2 DS 2 -VASc, and R 2 CHADS 2 as predictors of reduced LAFI, defined as a binary outcome divided at the median. Although we adjusted multivariate regressions for baseline clinical variables with p <0.10, we did not include any binary variables of the component co-morbidities of the risk scores because the risk scores are the primary predictor variables. We did include age as a continuous variable, because age is dichotomized or trichotomized in the risk scores. All analyses were conducted using STATA (version 12.2; StataCorp, College Station, Texas).
Results
The cohort consisted of 970 subjects (180 women). Baseline characteristics across tertiles of CHADS 2 are listed in Table 1 . The mean (±SD) CHADS 2 score was 1.7 ± 1.2; 464 (48%) had scores of 0 to 1, 407 (42%) had scores of 2 to 3, and 99 (10%) had scores of 4 to 6. There was no significant difference in sex, race, or AF prevalence across tertiles of CHADS 2 . Compared with those with low (0 to 1) CHADS 2 scores, subjects with intermediate (2 to 3) and high (≥4) scores were more likely to have higher pulse pressure, a history of hyperlipidemia, MI, coronary artery bypass graft, or percutaneous transluminal coronary angioplasty, and less likely to have normal weight or regular alcohol use. Tertiles of CHADS 2 were strongly associated with each component of the CHADS 2 index (CHF, hypertension, older age, diabetes, and stroke/TIA). Increase in tertile of CHADS 2 was strongly associated with increase in LV mass index, diastolic dysfunction, pulmonary artery systolic pressure, and inducible ischemia.
| Variable | CHADS 2 Score | ANOVA/χ 2 | Linear Trend | ||
|---|---|---|---|---|---|
| 0–1 (n = 464) | 2–3 (n = 407) | 4–6 (n = 99) | p Value | p Value | |
| Age (yrs) | 63.7 ± 9.7 | 68.7 ± 11.4 | 72.0 ± 10.4 | 0.02 | <0.001 |
| Women | 89 (19.2) | 78 (19.2) | 13 (13) | 0.34 | 0.30 |
| White | 296 (63.9) | 231 (56.8) | 52 (53) | 0.14 | 0.01 |
| African-American | 67 (14.5) | 77 (18.9) | 16 (16) | ||
| Hispanic | 37 (8.0) | 39 (9.6) | 10 (10) | ||
| Asian | 52 (11.2) | 42 (10.3) | 17 (17) | ||
| Other | 11 (2.4) | 18 (4.4) | 4 (4) | ||
| Hypertension | 239 (51.5) | 370 (90.9) | 90 (91) | <0.001 | <0.001 |
| Diabetes mellitus | 26 (5.6) | 186 (45.7) | 54 (55) | <0.001 | <0.001 |
| Hyperlipidemia | 284 (61.5) | 285 (70.4) | 76 (78) | 0.001 | <0.001 |
| MI | 224 (48.3) | 236 (58.0) | 68 (69) | <0.001 | <0.001 |
| Coronary artery bypass graft/percutaneous transluminal coronary angioplasty | 255 (55.0) | 250 (61.6) | 63 (64) | 0.08 | 0.03 |
| Stroke/TIA | 0 (0) | 52 (12.8) | 88 (89) | <0.001 | <0.001 |
| Heart failure | 12 (2.6) | 115 (28.3) | 50 (51) | <0.001 | <0.001 |
| Peripheral artery disease | 31 (6.7) | 36 (8.9) | 13 (13) | 0.09 | 0.03 |
| Body mass index (kg/m 2 ) | |||||
| <25 | 138 (29.7) | 98 (24.1) | 20 (20) | 0.03 | 0.06 |
| 25–29.9 | 188 (40.5) | 160 (39.3) | 51 (52) | ||
| ≥30 | 138 (29.7) | 149 (36.6) | 28 (28) | ||
| Smoking | |||||
| Never | 155 (33.4) | 117 (28.8) | 29 (29) | 0.01 | 0.92 |
| Past | 201 (43.3) | 224 (55.0) | 51 (52) | ||
| Current | 108 (23.3) | 66 (16.2) | 19 (19) | ||
| Regular alcohol use | 158 (34.1) | 103 (25.4) | 19 (19) | 0.002 | <0.001 |
| Systolic BP (mm Hg) | 129.5 ± 19.1 | 136.5 ± 21.6 | 135.6 ± 22.5 | <0.001 | <0.001 |
| Diastolic BP (mm Hg) | 75.0 ± 10.7 | 74.6 ± 11.4 | 73.5 ± 12.7 | 0.47 | 0.10 |
| Pulse pressure (mm Hg) | 54.4 ± 14.8 | 61.8 ± 16.8 | 62.0 ± 16.5 | <0.001 | <0.001 |
| Heart rate (beats/min) | 68.0 ± 12.3 | 68.6 ± 13.0 | 69.0 ± 12.4 | 0.68 | 0.48 |
| AF | 18 (3.9) | 19 (4.7) | 8 (8) | 0.20 | 0.11 |
| Aspirin | 326 (72.1) | 312 (76.9) | 64 (65) | 0.035 | 0.736 |
| β blocker | 244 (54.0) | 256 (63.1) | 61 (62) | 0.022 | 0.018 |
| ACE-I/ARB | 179 (39.6) | 258 (63.6) | 59 (60) | <0.001 | <0.001 |
| Statin | 269 (59.5) | 276 (68.0) | 77 (78) | 0.001 | <0.001 |
| Loop diuretic | 36 (7.8) | 90 (22.1) | 33 (33) | <0.001 | <0.001 |
| Thiazide | 47 (10.1) | 69 (17.0) | 14 (14) | 0.013 | 0.024 |
| LAFI | 42.8 ± 18.1 | 37.8 ± 19.1 | 36.7 ± 19.4 | <0.001 | <0.001 |
| Left ventricular mass index (g/m 2 ) | 91.7 ± 23.0 | 102.8 ± 27.9 | 106.6 ± 28.6 | <0.001 | <0.001 |
| Left ventricular ejection fraction (%) | 62.6 ± 8.5 | 60.8 ± 10.7 | 61.5 ± 9.2 | 0.02 | 0.04 |
| LA volume index (ml/m 2 ) | 31.3 ± 10.7 | 35.0 ± 12.9 | 34.2 ± 13.3 | <0.001 | <0.001 |
| Diastolic dysfunction | |||||
| None | 278 (68.5) | 204 (55.9) | 47 (53) | 0.002 | <0.001 |
| Impaired relaxation | 91 (22.4) | 104 (28.5) | 29 (33) | ||
| Pseudonormal/restrictive | 37 (9.1) | 57 (15.6) | 12 (14) | ||
| Pulmonary artery systolic pressure (mm Hg) | 29.9 ± 7.5 | 33.7 ± 9.8 | 33.9 ± 8.7 | <0.001 | <0.001 |
| Inducible ischemia present | 80 (18.2) | 111 (30.3) | 28 (34) | <0.001 | <0.001 |
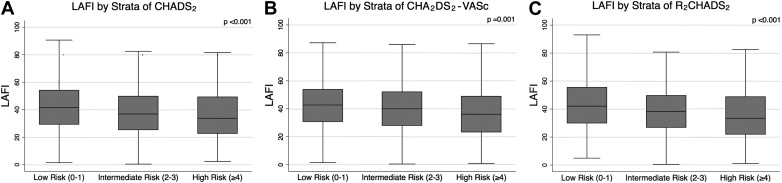
Mean LAFI was 40.1 ± 18.8. LAFI values across tertiles of CHADS 2 , CHA 2 DS 2 -VASc, and R 2 CHADS 2 are shown in Figure 1 . Subjects with baseline AF had lower LAFI compared with those without AF (12.4 vs 41.4). Increase in tertile of all 3 risk indices was significantly associated with decrease in mean LAFI (CHADS 2 score 0 to 1: 42.8 ± 18.1, score 2 to 3: 37.8 ± 19.1, score 4 to 6: 36.7 ± 19.4, p <0.001; CHA 2 DS 2 -VASc score 0 to 1: 43.4 ± 16.4, score 2 to 3: 41.0 ± 18.1, score ≥4: 37.3 ± 20.4, p = 0.001; R 2 CHADS 2 score 0 to 1: 44.0 ± 17.9, score 2 to 3: 38.8 ± 17.7, score ≥4: 35.6 ± 20.6, p <0.001).
Linear regression analyses of tertiles of CHADS 2, CHA 2 DS 2 -VASc, and R 2 CHADS 2 as predictors of LAFI are listed in Table 2 . When compared with the lowest tertile, intermediate and high tertiles of CHADS 2 were strongly associated with lower LAFI after adjustment for age, sex, race, systolic BP, hyperlipidemia, MI, revascularization, body mass index, smoking, and alcohol use (score 2 to 3: beta −4.10, p = 0.002, score 4 to 6: beta −5.24, p = 0.02). There were similar associations between tertiles of CHA 2 DS 2 -VASc (score 2 to 3: beta −2.66, p = 0.16; score ≥4: beta −5.58, p = 0.02) and R 2 CHADS 2 (score 2 to 3: beta −4.57, p = 0.001; score ≥4: beta −6.69, p <0.001) with LAFI. Linear regression analyses of risk scores as continuous predictors of LAFI are listed in Table 2 . For every 1 point increase in CHADS 2 , CHA 2 DS 2 -VASc, and R 2 CHADS 2 scores, the LAFI decreased by 4.0%, 3.1%, and 3.8%, respectively.
| Unadjusted | Adjusted ∗ | |||
|---|---|---|---|---|
| β (SE) | p Value | β (SE) | p Value | |
| CHADS 2 | ||||
| 2–3 | −4.96 (1.27) | <0.001 | −4.10 (1.33) | 0.002 |
| 4–6 | −6.10 (2.07) | 0.003 | −5.24 (2.17) | 0.02 |
| Per point increase | −1.88 (0.49) | <0.001 | −1.59 (0.53) | 0.003 |
| CHA 2 DS 2 -VASc | ||||
| 2–3 | −2.40 (1.74) | 0.17 | −2.66 (1.89) | 0.16 |
| ≥4 | −6.10 (1.81) | 0.001 | −5.58 (2.35) | 0.02 |
| Per point increase | −1.41 (0.38) | <0.001 | −1.22 (0.51) | 0.02 |
| R 2 CHADS 2 | ||||
| 2–3 | −5.21 (1.37) | <0.001 | −4.57 (1.42) | 0.001 |
| ≥4 | −8.39 (1.54) | <0.001 | −6.69 (1.77) | <0.001 |
| Per point increase | −1.85 (0.35) | <0.001 | −1.50 (0.40) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


