Mounting evidence suggests that statins possess antiarrhythmic properties and inhibit atrial fibrillation (AF). The goal of this study was to evaluate the relation between statin use and new-onset AF in a large cohort of patients with coronary artery disease. We identified all Medicare beneficiaries ≥65 years old who had been hospitalized for acute myocardial infarction or coronary revascularization from 1995 to 2004 and participated in 1 of 2 government-sponsored medication benefit programs. Patients with a history of AF before and during hospitalization were excluded. This yielded a cohort of 29,088. The incidence of new AF was compared between patients who were (n = 8,450) and were not (n = 20,638) prescribed statins within 1 month of hospital discharge after their cardiac event. New-onset AFs within 5 and 10 years were 32.6% and 51.2%, respectively, in patients who received statins compared to 38.3% and 58.0% in patients who did not receive statins (unadjusted hazard ratio 0.82, 95% confidence interval 0.78 to 0.86). Multivariable analysis controlling for demographic and clinical confounders indicated that statin use independently decreased the risk of developing new-onset AF compared to nonusers (adjusted hazard ratio 0.90, 95% confidence interval 0.85 to 0.94). Adjustment for propensity-score and health-seeking behaviors yielded nearly identical results. In conclusion, statin therapy initiated within 1 month after hospital discharge is independently associated with a decrease in the risk of new-onset AF after myocardial infarction or coronary revascularization. These findings lend support to the antiarrhythmic effects of statins and suggest another benefit for their use in patients with coronary artery disease.
Considerable evidence exists linking inflammatory processes and atrial fibrillation (AF). Inflammation may serve as a trigger for the initiation of AF, and through atrial electrophysiological and structural remodeling, create the substrate for the perpetuation of AF. Inflammatory changes have been detected in atrial biopsy specimens of patients with AF, and markers of inflammation such as C-reactive protein are higher in patients with AF. Accordingly, treatments that decrease inflammation may be associated with a decrease in the incidence of AF. Statins have antioxidant and antisympathetic activities that may help prevent electrical remodeling and direct antiarrhythmic effects through cell membrane ion channel stabilization. Although small studies have suggested that statins may prevent or attenuate AF, particularly in the postoperative period after cardiothoracic surgery, the impact of statin therapy in the primary prevention of AF outside the hospital setting has not been adequately evaluated. Therefore, we sought to assess the association between statin therapy and new-onset AF after hospitalization for treatment of coronary artery disease (CAD).
Methods
We assembled a cohort of Medicare beneficiaries with CAD by linking Medicare files describing all clinical encounters to complete medication-use data from the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE) and the New Jersey Pharmaceutical Assistance to the Aged and Disabled (PAAD) programs. During the period studied, PACE and PAAD provided prescription drug benefits to lower middle-income patients ≥65 years of age whose yearly earnings were above the threshold to qualify them for Medicaid. Participants paid copayments from $5 to $10 per prescription without any deductibles. The programs cover all medications that require a prescription and do not restrict which medications can be prescribed (i.e., the programs do not use formularies, preferred drug lists, or previous authorization programs).
We assembled data from PACE, PAAD, and Medicare into a relational database consisting of claims for all filled prescriptions, procedures, physician encounters, hospitalizations, long-term care admissions, and deaths for the patients in this cohort. These data sources have been used extensively to study population-based medication use and health outcomes. All traceable patient-specific identifying factors were transformed into anonymous coded study numbers to protect subjects’ privacy. This study was approved by the institutional review board of the Brigham and Women’s Hospital (Boston, Massachusetts).
We included all patients who were discharged alive from the hospital after admission for active CAD from January 1, 1995, to December 31, 2004. This included patients admitted for myocardial infarction ( International Classification of Diseases, Ninth Revision , 410.01 to 410.91 or 411), percutaneous coronary intervention ( International Classification of Diseases, Ninth Revision , 36.01 to 36.09), or coronary artery bypass graft surgery (CABG; International Classification of Diseases, Ninth Revision , 36.1× or 36.2×). We excluded patients who died or were readmitted to hospital within 30 days after hospital discharge, patients who were not active users of either drug benefit program, and patients who received prescriptions for cerivastatin because this drug was withdrawn from the market. We also excluded patients who had a diagnosis of AF before or during the index hospitalization, as documented on inpatient or outpatient codes ( International Classification of Diseases, Ninth Revision , 427.31). Validation studies have demonstrated that this code from the International Classification of Diseases, Ninth Revision , has a specificity of 99% and positive predictive value of 97% for the diagnosis of AF. Thirty days after the date of hospital discharge was considered the index date for the study analysis (i.e., start of follow-up). Follow-up terminated December 31, 2005.
We determined patient co-morbidities by searching physician service claims and hospitalization records for relevant diagnostic codes in the 1-year period before the index date. In this manner, the following characteristics were identified: age at index date, year of hospitalization, gender, race, length of hospital stay, previous myocardial infarction or acute coronary syndrome, hypertension, diabetes mellitus, congestive heart failure, stroke, peripheral vascular disease, previous CABG, previous percutaneous coronary intervention, and chronic kidney disease. We assessed statin use in the 1-year period before the index CAD hospitalization and within 30 days after the discharge date. We also determined the use of the following concurrent medications in the 1-year period before and 30 days after CAD hospitalization: amiodarone, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, β blockers, calcium channel blockers, clopidogrel, fibrates, diuretics, nitrates, digoxin, and warfarin. Hospitals accredited by the Association of American Medical Colleges were classified as teaching hospitals. All other hospitals were classified as nonteaching hospitals.
Information regarding tobacco use and alcohol dependence was not available within the study database. To supplement our analyses, we used data from the 2004 Medicare Current Beneficiary Survey, a nationwide in-home survey. Using previously described methods, we restricted our analysis to community-dwelling subjects ≥65 years old who used ≥1 medication during the study period. We compared current smoking rates and alcohol dependence in patients reporting the use versus nonuse of statins in 2004. In this analysis, current smoking rates were similar between statin users and nonusers (8.8% vs 9.0%, statin users vs nonusers, p = 0.75). Alcohol dependence was also similar between statin users and nonusers (2.6% vs 2.2%, statin users vs nonusers, p = 0.22).
Our primary outcome was new-onset AF in patients with no documented history of AF before or during the index hospitalization. We compared rates of this outcome for patients who did and did not fill a statin prescription within 30 days of hospital discharge. Follow-up began on the index date (i.e., 30 days after hospital discharge). AF was defined as any documented diagnosis of AF occurring as an outpatient or as an inpatient during a subsequent hospital admission. Baseline characteristics between statin users and nonusers were compared using Student’s t tests, Fisher’s exact tests, or chi-square trend tests, as appropriate. Statistical significance was defined as a p value <0.05.
Univariate estimates of new-onset AF were determined for statin users and nonusers using the Kaplan-Meier method, and groups were compared using a log-rank test. Patients were censored at the end of follow-up or if they developed the outcome of interest. We then used multivariable Cox proportional hazards models to adjust for potentially important differences between statin users and nonusers. Factors of clinical relevance incorporated into the models were age, gender, race, year of index hospitalization, treatment in a teaching hospital, length of hospital stay, history of peripheral vascular disease, hypertension, congestive heart failure, chronic kidney disease, previous stroke, previous myocardial infarction, previous coronary revascularization (percutaneous coronary intervention or CABG), diabetes mellitus, medication use in the 1-year period before hospital admission (statin, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, β blocker, or amiodarone), and medication use within 30 days after hospital discharge (angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, β blocker, calcium channel blocker, digoxin, or amiodarone). Hazard ratios (HRs) are reported with SEs or 95% confidence intervals (CIs). All analyses were performed using SAS 8.2 (SAS Institute, Cary, North Carolina).
We repeated our analyses in each CAD subgroup (myocardial infarction, percutaneous coronary intervention, or CABG) to test whether the impact of statins on rates of AF differed by inclusion criteria. We also excluded outpatient AF visits from our outcome measurements to assess the association between statins and AF hospitalization. Because patients who use statins and other preventive medications may be more likely to adopt other health-seeking behaviors that affect clinical outcomes, we repeated our analyses by including covariates in our multivariable Cox models that adjust for the “healthy-user effect.” These healthy-user markers were assessed during the 1-year period before the index date and included influenza vaccination, pneumococcal vaccination, mammography, bone mineral densitometry, fecal occult blood testing, and prostate-specific antigen testing. We then performed a propensity score-adjusted analysis. A propensity score for statin use after hospitalization was developed using logistic regression with the following covariates: teaching hospital, age, gender, white race, year of index hospitalization, history of congestive heart failure, diabetes mellitus, peripheral vascular disease, stroke, and statin use in the 1-year period before the index hospitalization. Statin users were matched 1:1 to nonusers based on propensity score. Univariate and multivariable analyses were then repeated within this propensity-matched cohort.
Results
Our cohort consisted of 29,088 patients who had no previous AF and were admitted to the hospital with a myocardial infarction (n = 12,235) or for percutaneous coronary intervention (n = 11,722) or CABG (n = 5,131). Mean follow-up for the entire cohort was 3.8 ± 3.0 years (maximum 10.9). Within 1 month of hospital discharge, 29.0% of patients were prescribed statins.
Table 1 lists characteristics of patients who did and did not fill prescriptions for statins within 1 month of hospital discharge. Statin users were more likely than nonusers to have received statins before hospitalization and to have filled prescriptions for other cardiac medications, including angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, β blockers, and clopidogrel before and after hospitalization (all p values <0.05). Although statin users were more likely to have diabetes mellitus or hypertension, statin nonusers were more likely to be older, have longer hospital stays, and have congestive heart failure or a history of stroke (all p values <0.05). Statin users were more likely to have been admitted to a teaching hospital (p <0.05). Propensity-matching substantially improved the balance between patient- and hospital-related characteristics.
| Characteristic | Entire Study Cohort | Propensity-Matched Cohort | ||
|---|---|---|---|---|
| Statin Nonusers | Statin Users | Statin Nonusers | Statin Users | |
| (n = 20,638) | (n = 8,450) | (n = 6,743) | (n = 6,743) | |
| Myocardial infarction | 47.7% | 17.1% | 34.1% | 29.2% |
| Percutaneous coronary intervention | 34.4% | 28.3% | 44.7% | 54.5% |
| Coronary bypass | 17.9% | 54.6% | 21.1% | 16.3% |
| Age (years), mean ± SD | 78.8 ± 7.1 | 76.6 ± 6.4 ⁎ | 76.8 ± 6.5 | 76.9 ± 6.4 |
| Women | 72.2% | 74.1% ⁎ | 74.5% | 74.1% |
| White | 91.3% | 90.6% ⁎ | 91.2% | 91.0% |
| Previous myocardial infarction | 23.5% | 22.5% | 22.7% | 22.3% |
| Heart failure | 53.4% | 42.0% ⁎ | 45.4% | 43.8% |
| Stroke | 8.1% | 5.7% ⁎ | 5.4% | 5.8% |
| Peripheral vascular disease | 5.1% | 5.3% | 5.7% | 5.2% |
| Hypertension | 82.0% | 88.0% ⁎ | 87.0% | 87.7% |
| Diabetes mellitus | 43.4% | 45.4% ⁎ | 46.6% | 45.6% |
| Chronic kidney disease | 27.4% | 25.6% ⁎ | 29.6% | 25.0% ⁎ |
| Previous coronary bypass | 0.5% | 0.8% ⁎ | 0.6% | 0.8% |
| Previous percutaneous coronary intervention | 3.0% | 3.7% | 4.1% | 3.4% ⁎ |
| Prehospital medication use | ||||
| Previous statin | 17.9% | 60.7% ⁎ | 50.4% | 52.4% ⁎ |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker | 48.0% | 54.9%* | 50.5% | 54.0%* |
| Clopidogrel | 20.2% | 41.6% ⁎ | 32.3% | 40.1% ⁎ |
| β blocker | 54.1% | 69.0% ⁎ | 59.3% | 67.8% ⁎ |
| Calcium channel blocker | 52.9% | 50.4% ⁎ | 49.4% | 50.5% |
| Digoxin | 12.6% | 9.2% ⁎ | 11.0% | 9.0% ⁎ |
| Diuretics | 10.2% | 12.1% ⁎ | 12.0% | 11.5% |
| Fibrate | 3.6% | 3.7% | 4.6% | 3.7% ⁎ |
| Nitrates | 64.6% | 64.3% | 60.8% | 63.9% ⁎ |
| Amiodarone | 0.2% | 0.3% | 0.2% | 0.3% |
| Warfarin | 8.0% | 6.5% ⁎ | 5.9% | 6.5% |
| Posthospital medication use | ||||
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker | 28.1% | 42.1%* | 27.3% | 42.2%* |
| Clopidogrel | 15.7% | 39.2% ⁎ | 25.5% | 37.6% ⁎ |
| β blocker | 37.3% | 60.0% ⁎ | 40.0% | 59.8% ⁎ |
| Calcium channel blocker | 21.8% | 25.0% ⁎ | 19.0% | 24.9% ⁎ |
| Digoxin | 7.8% | 7.0% ⁎ | 6.6% | 6.8% |
| Diuretics | 2.9% | 4.8% ⁎ | 3.5% | 4.6% ⁎ |
| Fibrate | 1.5% | 1.0% ⁎ | 1.8% | 0.9% ⁎ |
| Nitrates | 40.8% | 45.2% ⁎ | 33.4% | 45.7% ⁎ |
| Amiodarone | 1.0% | 1.6% ⁎ | 1.1% | 1.2% |
| Warfarin | 5.4% | 5.2% | 3.4% | 4.9% ⁎ |
| Hospital characteristics | ||||
| Teaching hospital | 61.4% | 64.8% ⁎ | 65.6% | 65.0% |
| Length of stay (days), mean ± SD | 7.6 ± 6.1 | 5.7 ± 4.2 ⁎ | 6.9 ± 5.9 | 5.7 ± 4.1 ⁎ |
| Mean follow-up (years) | 3.9 ± 3.1 | 3.6 ± 2.7 ⁎ | 2.8 ± 2.5 | 3.1 ± 2.6 ⁎ |
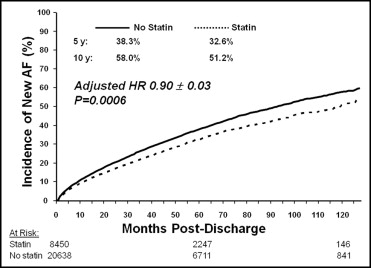
New-onset AF in patients who received statins occurred in 10.6%, 32.6%, and 51.2%, at 1 year, 5 years, and 10 years, respectively. Corresponding rates in patients who did not fill a statin prescription were 12.9%, 38.3%, and 58.0% ( Figure 1 ). On univariate analysis, statin users were significantly less likely to develop new-onset AF than nonusers (HR 0.82, 95% CI 0.78 to 0.86). After adjusting for patient- and hospital-related characteristics, statin use was independently associated with a lower risk of new-onset AF (HR 0.90, 95% CI 0.85 to 0.96; Table 2 ). Similar unadjusted and adjusted results were seen in each of the CAD subgroups ( Table 3 ).