Vascular complications (VCs) occur in 3% to 8% of percutaneous coronary interventions (PCIs). However, only a portion of patients who experience VCs bleed significantly. The aim of this study was to assess the covariates associated with the amount of blood loss in patients experiencing postprocedural VCs as well as the effect of the degree of blood loss on long-term mortality. Overall, 7,718 unselected patients who underwent PCI through femoral access were evaluated. Those experiencing VCs were identified and stratified with regard to the degree of hematocrit (HCT) decrease after the procedure. In total, 444 patients (5.8%) had VCs. Compared to those without VCs, patients with VCs were older and had more extensive co-morbidities. Severe blood loss was most frequent in those who had vascular perforation requiring surgical repair or in those who had retroperitoneal bleeding. Overall, <25% of patients with hematoma had severe blood loss. The raw 1-year mortality was doubled in patients with minimal or moderate HCT decrease and was tripled in those with severe decreases in HCT. Similarly, the rate of definite stent thrombosis was tripled in patients with VCs and moderate or severe decreases in HCT. After adjustment, only patients with VCs and the greater HCT decreases had an increased risk for death at 1 year (hazard ratio 1.80, 95% confidence interval 1.03 to 3.14). Independent predictors of severe HCT decrease included age, female gender, glycoprotein IIb/IIIa inhibitor use, and activated clotting time peak. Bivalirudin and closure devices were independently associated with less frequent severe HCT decrease. In conclusion, VCs do not entail an increased risk for death at 1 year unless associated with severe blood loss. The use of bivalirudin and closure devices seems to reduce the risk for such complications.
Arterial access-related vascular complications (VCs) occur in 3.5% to 8.4% of percutaneous coronary interventions (PCIs) performed using the femoral approach. Importantly, VCs have been associated with increased mortality at 30 days and at 1 year. It is relatively easy to understand why VCs are associated with increased mortality at 30 days, but it is more difficult to explain why the relation persists at 1 year. A number of mechanisms have been proposed to explain this association: (1) more severe cardiovascular disease in patients with VCs, (2) hemodynamic compromise or myocardial injury caused by VC-related hemorrhage, (3) putative adverse effects of red blood cell transfusion on late survival, and (4) the need for abrupt cessation of antithrombotic therapy. However, some patients experiencing VCs do not bleed significantly. Few data are available regarding the predictors of bleeding and its magnitude. Furthermore, the effect of the amount of blood loss on long-term mortality has not been addressed in detail. This study was designed to address these unanswered questions.
Methods
We evaluated 7,718 consecutive patients who underwent PCI via femoral access at a single center from 2003 to 2009 for whom 1-year follow-up was available. Indications for PCI included stable angina pectoris, unstable angina pectoris, and myocardial infarction. Patients with cardiogenic shock at presentation were not included. All patients gave written consent for the procedure. The study was approved by our local institutional review board.
All patients received aspirin 325 mg and clopidogrel 300 to 600 mg (at the operator’s discretion) before the procedure. During PCI, anticoagulation regimens were chosen by the operator and included either unfractionated heparin targeted to achieve and maintain an activated clotting time of 250 to 300 seconds (200 to 250 seconds if glycoprotein IIb/IIIa inhibitors were used) or bivalirudin 0.75 mg/kg (bolus) followed by an infusion of 1.75 mg/kg per hour for the duration of the procedure. Adjunctive glycoprotein IIb/IIIa receptor inhibitors were used at the discretion of the operator. After the procedure, aspirin 325 mg was prescribed indefinitely, and clopidogrel 75 mg was prescribed for ≥1 month in patients receiving bare-metal stents and for 6 to 12 months in patients with acute coronary syndromes or those receiving drug-eluting stents.
Femoral access was routinely used during the study period. Vascular access was managed irrespectively of the anticoagulant agent chosen. When vascular closure devices were used, a femoral angiogram was obtained before their placement, and ambulation was then allowed 2 hours later. When the arterial access was not sealed in the laboratory, the activated clotting time was checked hourly until <200 seconds, and the sheath was then removed. Ambulation was initiated 6 hours after removal. After the procedure and before discharge, access site evaluation was routinely performed and recorded.
VCs were defined as any of the following at the access site: (1) hematoma ≥5 cm as defined in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial, (2) arteriovenous fistulas, (3) vascular perforation or laceration requiring surgery, (4) pseudoaneurysm, (5) retroperitoneal bleeding (confirmed by an imaging test), and (6) acute limb ischemia.
Patients with VCs were then stratified by the amount of hematocrit (HCT) decrease after the procedure. Because the magnitude of decrease was not normally distributed, patients with VCs were stratified into quartiles. Therefore, 4 groups of patients with VCs were created: (1) patients with severe HCT decreases (highest quartile; HCT decrease ≥9.2), (2) those with moderate HCT decreases (second quartile; HCT decrease ≥5.8 to <9.2), (3) those with minimal HCT decreases (third quartile; HCT decrease ≥3 to <5.8), and (4) those without HCT decreases (lowest quartile; HCT decrease <3).
All data management and analysis were performed at a dedicated coordinating center (Data Center, Cardiovascular Research Institute, Washington, District of Columbia). Independent research personnel unaware of the study objectives abstracted from medical records prespecified demographic, clinical, and procedural data as well as access site VCs and entered these data into a database. Clinical follow-up was performed at 1, 6, and 12 months by telephone contact or office visit. Source documentation of all clinical events (including death) was obtained. A committee of independent physicians not involved in the procedures adjudicated the nature of the event.
Statistical analysis was performed using SAS version 9.1 (SAS Institute, Inc., Cary, North Carolina). Continuous variables are expressed as mean ± SD unless otherwise noted. Categorical variables are expressed as frequencies and group percentages. Baseline characteristics were compared using chi-square tests for categorical variables and analysis of variance or Kruskal-Wallis tests for continuous variables. One-year outcomes were compared using log-rank tests and described by Kaplan-Meier estimates.
To test the independent effect of the level of blood loss in patients with VCs on mortality at 1 year, a multivariate Cox regression model was constructed. Clinically relevant variables with p values <0.05 on individual analysis were introduced in the model. It included quartile of HCT decrease, age, gender, systemic hypertension, diabetes mellitus, peripheral vascular disease, chronic renal insufficiency, history of surgical revascularization, symptomatic heart failure, acute myocardial infarction presentation, baseline HCT, left main coronary artery PCI, number of lesions, use of an intra-aortic balloon pump, bivalirudin anticoagulation during PCI, and use of closure devices to manage the access site. The proportional-hazards assumption was assessed by Kolmogorov-type supremum test.
To identify independent variables associated with VCs and severe bleeding, multivariate logistic regression analysis was used. Variables with significant univariate p values (<0.05) and clinical relevance were included (age, gender, body mass index, chronic renal insufficiency, peripheral vascular disease, acute myocardial infarction, maximum sheath size in the procedure, use of glycoprotein IIb/IIIa inhibitors, length of procedure in minutes, bivalirudin anticoagulation, peak of intraprocedural activated clotting time, use of closure devices, and intra-aortic balloon pump use).
Results
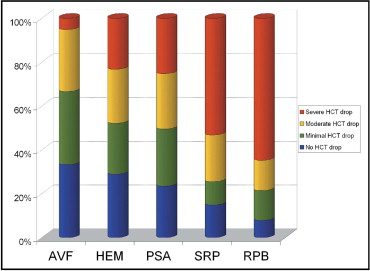
From the study cohort of 7,718 patients, a total of 444 (5.8%) experienced VCs. Besides the access-site bleeding, 8 patients with VCs (1.9%) had gastrointestinal bleeding, whereas only 33 (0.5%) in the group without VCs did. Hematomas ≥5 cm were found in 345 patients (4.5%), arteriovenous fistulas in 39 (0.5%), and pseudoaneurysms in 115 (1.5%). Surgical repair was required in 47 (0.6%), and retroperitoneal hemorrhage was found in 51 (0.6%). No patient had distal limb ischemia. Figure 1 demonstrates that severe blood loss was most frequent in those who required surgical repair or who had retroperitoneal bleeding. Severe bleeding was present in <25% patients with femoral hematomas or pseudoaneurysms. Patients with arteriovenous fistulas rarely had severe blood loss.
Baseline characteristics are listed in Table 1 . Patients with VCs were older and more frequently female. They had more extensive co-morbidities and more often presented with acute myocardial infarction (p <0.001). Procedural details are listed in Table 2 . Four were strongly associated with a higher incidence of VCs: glycoprotein IIb/IIIa inhibitor use, higher intraprocedural activated clotting time peak, longer procedure time, and the use of an intra-aortic balloon pump (all p values <0.005). In all 4 instances, especially strong associations with severe HCT decrease were observed. Two procedural features were associated with fewer VCs with severe blood loss: the use of bivalirudin in lieu of heparin and the use of closure devices (both p values <0.001). Bivalirudin use was less frequent in patients with severe blood loss than in those with VCs but with lesser decreases (p <0.05). Patients with VC receiving closure devices had a 5.8% median HCT decrease (interquartile range 2.2% to 8.5%), whereas those who underwent manual compression had a 6.1% median HCT decrease (interquartile range 3.4% to 9.8%) (p = 0.04).
| Variable | No VCs (n = 7,274) | VCs and No HCT Decrease (n = 125) | VCs and Minimal HCT Decrease (n = 109) | VCs and Moderate HCT Decrease (n = 104) | VCs and Severe HCT Decrease (n = 106) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 65.0 ± 11.7 | 69.0 ± 10.6 | 67.8 ± 14.1 | 70.9 ± 11.9 | 70.1 ± 12.0 | <0.001 |
| Men | 4,829 (66.4%) | 73 (58.4%) | 45 (41.3%) | 49 (47.1%) | 45 (42.5%) | <0.001 |
| European American | 5,014 (68.9%) | 91 (72.8%) | 72 (66.1%) | 67 (64.4%) | 81 (76.4%) | 0.283 |
| African American | 1,675 (23.0%) | 18 (14.4%) | 24 (22.0%) | 26 (25.0%) | 19 (17.9%) | 0.138 |
| Body mass index (kg/m 2 ) | 29.7 ± 6.3 | 28.9 ± 7.2 | 28.4 ± 6.8 | 29.1 ± 6.6 | 28.3 ± 6.6 | 0.015 |
| Systemic hypertension ⁎ | 6,145 (84.8%) | 107 (85.6%) | 86 (78.9%) | 90 (86.5%) | 88 (83.8%) | 0.515 |
| Diabetes mellitus | 2,526 (35.0%) | 48 (39.0%) | 34 (31.5%) | 38 (36.5%) | 33 (31.1%) | 0.686 |
| Insulin-requiring diabetes | 791 (11.0%) | 18 (14.6%) | 9 (8.3%) | 13 (12.5%) | 10 (9.4%) | 0.565 |
| Hyperlipidemia | 6,370 (88.3%) | 104 (85.2%) | 88 (81.5%) | 92 (89.3%) | 86 (82.7%) | 0.072 |
| Previous coronary artery bypass graft | 1,440 (19.9%) | 23 (18.5%) | 21 (19.4%) | 20 (19.4%) | 16 (15.2%) | 0.814 |
| Peripheral vascular disease | 1,113 (15.4%) | 26 (21.3%) | 23 (21.3%) | 19 (19.2%) | 23 (21.9%) | 0.044 |
| Chronic renal failure | 878 (12.1%) | 23 (18.5%) | 20 (18.5%) | 23 (22.5%) | 16 (15.4%) | <0.001 |
| Current smokers | 1,404 (19.3%) | 27 (21.6%) | 27 (24.8%) | 15 (14.4%) | 18 (17.0%) | 0.346 |
| Acute myocardial infarction | 952 (13.1%) | 29 (23.2%) | 15 (13.8%) | 24 (23.1%) | 28 (26.4%) | <0.001 |
| HCT at baseline | 39.8 ± 6.5 | 35.6 ± 6.5 | 37.1 ± 5.1 | 38.2 ± 5.1 | 40.0 ± 4.7 | <0.001 |
⁎ History of hypertension diagnosed and/or treated with medication or currently being treated with diet and/or medication by a physician.
| Variable | No VCs (n = 7,274) | VCs and No HCT Decrease (n = 125) | VCs and Minimal HCT Decrease (n = 109) | VCs and Moderate HCT Decrease (n = 104) | VCs and Severe HCT Decrease (n = 106) | p Value |
|---|---|---|---|---|---|---|
| Bivalirudin use | 5,559 (76.4%) | 83 (66.4%) | 78 (71.6%) | 71 (68.3%) | 64 (60.4%) | <0.001 |
| Peak activated clotting time (seconds) | 338.3 ± 72 | 352.2 ± 107 | 347.5 ± 89 | 351.2 ± 82 | 354.8 ± 87 | 0.005 |
| Glycoprotein IIb/IIIa inhibitors | 674 (9.3%) | 17 (13.8%) | 18 (16.7%) | 18 (17.6%) | 21 (20.0%) | <0.001 |
| Intra-aortic balloon pump | 170 (2.4%) | 8 (6.5%) | 6 (5.6%) | 8 (7.8%) | 12 (11.4%) | <0.001 |
| Number of lesions PCI | 1.71 ± 1.6 | 1.56 ± 0.9 | 1.75 ± 0.9 | 1.78 ± 1.1 | 1.80 ± 1.2 | 0.766 |
| Procedure length (minutes) | 56.2 ± 35.2 | 65.3 ± 84.0 | 55.1 ± 26.6 | 59.5 ± 36.3 | 71.2 ± 82.7 | <0.001 |
| Maximum sheath size | 6.64 ± 1.37 | 6.70 ± 0.95 | 6.75 ± 0.89 | 6.76 ± 0.69 | 6.84 ± 0.98 | 0.014 |
| Closure devices | 4,680 (65.6%) | 55 (46.6%) | 41 (38.7%) | 37 (36.6%) | 39 (37.1%) | <0.001 |
| Target coronary vessel (lesion based) | ||||||
| Left main coronary artery | 242 (1.9%) | 5 (2.4%) | 7 (3.4%) | 7 (3.5%) | 4 (2.0%) | 0.264 |
| Left anterior descending coronary artery | 4,732 (37.1%) | 77 (37.4%) | 81 (39.7%) | 72 (35.8%) | 73 (36.5%) | 0.942 |
| Left circumflex coronary artery | 3,004 (23.5%) | 46 (22.3%) | 51 (25.0%) | 42 (20.9%) | 46 (23.0%) | 0.876 |
| Right coronary artery | 4,008 (31.4%) | 63 (30.6%) | 60 (29.4%) | 68 (33.8%) | 69 (34.5%) | 0.761 |
| Saphenous vein graft | 708 (5.5%) | 15 (7.3%) | 4 (2.0%) | 12 (6.0%) | 6 (3.0%) | 0.068 |
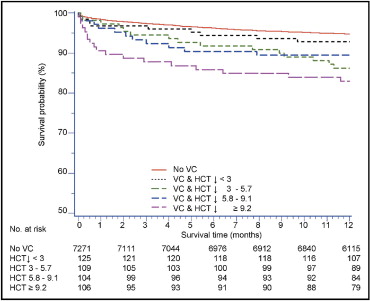
Outcomes by strata of HCT decrease are shown in Figure 2 and listed in Table 3 . Raw mortality was not significantly different in patients with VCs without HCT decreases. It was, however, associated with a nearly doubled mortality rate in the presence of minimal or moderate HCT decrease. In the quartile with the largest HCT decrease, the 1-year mortality was triple that seen when no VCs occurred. The association between mortality and severe HCT decrease was significant at all 3 time points (in hospital, 30 days, and 1 year; p <0.001 for all). After accounting for baseline differences, multivariate Cox regression analysis ( Figure 3 ) suggested that the occurrence of VCs alone was independently associated with 1-year mortality only when accompanied by a severe HCT decrease. When a severe decrease was encountered, the risk for 1-year mortality was increased by roughly 80% (hazard ratio 1.80, 95% confidence interval [CI] 1.03 to 3.14, p = 0.04).
| Variable | No VCs (n = 7,274) | VCs and No HCT Decrease (n = 125) | VCs and Minimal HCT Decrease (n = 109) | VCs and Moderate HCT Decrease (n = 104) | VCs and Severe HCT Decrease (n = 106) | p Value |
|---|---|---|---|---|---|---|
| In hospital | ||||||
| All-cause death | 1.2 | 2.4 | 2.8 | 2.9 | 7.5 | <0.001 |
| 30 days | ||||||
| All-cause death | 1.8 | 4.0 | 2.8 | 3.8 | 10.4 | <0.001 |
| Definite stent thrombosis | 0.5 | 0.0 | 0.9 | 1.9 | 2.8 | 0.010 |
| 1 year | ||||||
| All-cause death | 5.4 | 7.2 | 13.8 | 10.6 | 17.0 | <0.001 |
| Definite stent thrombosis | 0.8 | 0.0 | 0.9 | 2.9 | 2.8 | 0.017 |
| Target lesion revascularization | 5.6 | 2.5 | 6.7 | 6.1 | 6.4 | 0.634 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


