Systematic evaluation of left ventricular (LV) endocardial fibroelastosis (EFE) in the fetus has not been reported. The role of EFE in the pre- and postnatal evolution of hypoplastic left heart disease, and the implications of EFE for outcomes after prenatal intervention for fetal aortic stenosis with evolving hypoplastic left heart syndrome have also not been determined. A 4-point grading system (0-3) was devised for the assessment of fetal LV echogenicity, which was presumed to be due to EFE. Two reviewers independently graded EFE on the preintervention echocardiograms of fetuses treated with in utero aortic valvuloplasty for evolving hypoplastic left heart syndrome from 2000 to 2008. Intra- and interobserver reproducibility was determined for the EFE grade and characterization of related echocardiographic features. The relations among EFE severity, other left heart anatomic and physiologic variables, and postintervention outcomes were analyzed. The assessment and grading of EFE was possible for both observers in all 74 fetuses studied. By consensus, the EFE severity was grade 1 in 31 patients, grade 2 in 32, and grade 3 in 11. Fetuses with mild (grade 1) EFE had significantly greater maximum instantaneous aortic stenosis gradients (e.g., higher LV pressures) and less globular LV geometry than patients with grade 2 or 3 EFE on preintervention echocardiogram. The severity of EFE was not associated with the size of the aortic valve or LV. From preintervention to late gestation, the time-indexed change in LV end-diastolic volume was significantly greater in fetuses with grade 1 EFE than those with more severe EFE. Incorporation of EFE severity into our previously published threshold score improved the sensitivity and positive predictive value for the postnatal biventricular outcomes. In conclusion, echocardiographic grading of EFE is possible, with reasonable intra- and interobserver reliability in midgestation fetuses with evolving hypoplastic left heart syndrome. EFE severity corresponded to some indexes of left heart size, geometry, and function and with the probability of a biventricular outcome postnatally. Additional experience and external validation of the EFE grading scoring system are necessary.
Among midgestation fetuses with aortic stenosis (AS) and left ventricular (LV) dysfunction, some will progress to have hypoplastic left heart syndrome (HLHS) postnatally. In fetuses with this condition, which can be characterized as “evolving HLHS,” the LV endocardial surface is often echogenic, presumably because of endocardial fibroelastosis (EFE). In utero balloon dilation of the aortic valve has been shown to improve LV function and facilitate postnatal survival with a biventricular circulation in a subgroup of fetuses with evolving HLHS. Postnatally, EFE has been suspected on echocardiograms and demonstrated by magnetic resonance imaging, surgical inspection, and histopathologic examination in patients who underwent fetal intervention for evolving HLHS. An important unanswered question is the effect of EFE on the growth of the left heart, particularly the LV, after in utero aortic valvuloplasty. EFE, given its inelastic fibrotic properties, might restrict LV growth after in utero aortic valvuloplasty. However, it is difficult to assess the importance of EFE in the fetus or newborn with hypoplastic left heart disease, insofar as characterizing the extent and severity of EFE remains challenging. The accuracy and reliability of echocardiography for assessing EFE in newborns with AS is modest at best. Although qualitative assessment of fetal EFE by echocardiography has been reported, no published data are available regarding the reliability of systematic grading of fetal EFE. The aim of the present study was to assess the feasibility of diagnosing LV EFE in the midgestation fetus with AS and evolving HLHS, devise a grading scale for fetal LV echogenicity that is reasonably reliable, and determine whether preintervention LV EFE is associated with the anatomic characteristics of the LV or with LV growth or postnatal outcome after in utero aortic valvuloplasty.
Methods
The present study included all midgestation fetuses with predominant AS in which prenatal aortic valvuloplasty was attempted for evolving HLHS from 2001 to 2008 and with data available for review. Preintervention fetal echocardiograms were analyzed in a retrospective blinded fashion. Fetal interventions were considered technically successful if a balloon was inflated across the valve and there was improvement in anterograde color flow and/or new aortic regurgitation, as defined previously. For patients surviving to term or near term, postnatal outcomes were defined as biventricular from the time of birth (no period with univentricular palliation), biventricular circulation after initial univentricular palliation (stage I palliation and other univentricular palliative procedures later converted to biventricular circulation), and univentricular circulation.
Digital images from the fetal echocardiogram just before in utero aortic valvuloplasty were used for analysis. Echocardiography was performed using either a Philips 7500 (Philips Healthcare, Andover, Massachusetts) or an Acuson Siemens Sequoia 512 (Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania) machine, and the settings were adjusted to optimize image quality according to standard practice in our laboratory. The left heart dimensions and function were measured, as previously described. De-identified studies were reviewed by 2 fetal echocardiographers who were unaware of the postnatal outcome, with 2 reviews conducted 1 week apart by each reviewer. The studies were reviewed a third time by both reviewers, and a consensus assessment was made for each study variable.
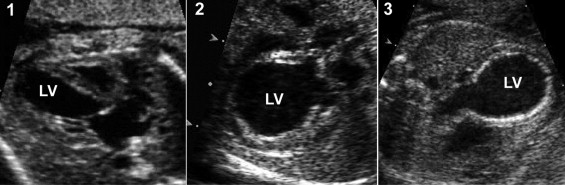
The term “EFE” is used in this report to refer to the echocardiographic findings of LV echogenicity outlined below. The following features related to EFE were assessed using the specified grading criteria. The components of the review were developed empirically on the basis of the previous experience of one of us (senior reviewer, WT). The examples and ranges of each component of the EFE are depicted in Figure 1 . No reference standard was used for comparison to the echocardiographic findings in the present study.
- 1
EFE severity was graded as 0, none; 1, mild (scattered echogenic spots within the LV, including the mitral valve [MV] papillary muscles); 2, moderate (noncontiguous echogenic patches throughout the LV); and 3, severe (contiguous echogenic lining of the LV; Figure 1 )
- 2
EFE on the ventricular septum, more prominent than the rest of the LV (0, no EFE; 1, EFE present but not more prominent than elsewhere in the LV; and 2, EFE present and more prominent)
- 3
Echogenic papillary muscles (1, yes; and 0, no)
- 4
Presence of an immobile, echogenic strand running between the MV or tension apparatus and the LV outflow tract (1, yes; and 0, no; Figure 2 )

Figure 2
Echocardiographic image from fetus with grade 2 EFE, with MV-LV outflow echogenic strand clearly shown (arrows).
The intra- and interobserver reproducibility of assessments of EFE severity, the relative extent of septal EFE, papillary muscle brightness, the presence of a MV strand, and the relative LV length were assessed by κ analysis, with a weighted κ analysis in the case of ordinal variables (in all such cases, 3 grades were used, which were weighted 0, 0.5, and 1). For assessment of intraobserver reproducibility, the 2 measurements by observer 1 were compared with each another and the 2 measurements by observer 2 were compared with each another. For interobserver analysis, the second set of assessments by observer 1 was compared with the second set of assessments by observer 2. The κ values are reported with standard errors. The relations between EFE severity and the other categorical and continuous anatomic variables were assessed using nonparametric testing, with Fisher’s exact test, chi-square analysis, or the Wilcoxon rank-sum test. After testing for normality, the preintervention left heart anatomic and functional variables were compared according to EFE severity as a dichotomous variable (mild or less vs greater than mild) using a 2-tailed t test. EFE severity was added to our previously published threshold score to determine whether a misclassification error could be corrected, and the specificity and positive predictive value of the threshold score improved.
Results
A total of 74 consecutive patients with AS and evolving HLHS underwent attempted prenatal aortic valvuloplasty and were included in the present study. Preintervention echocardiograms were performed using a Siemens (n = 41) or Philips (n = 33) machine. Assessment and grading of EFE was possible for both observers in all 74 cases. The intra- and interobserver variability analyses are summarized in Table 1 . By consensus, the EFE severity was grade 1 in 31 patients, grade 2 in 32, and grade 3 in 11. An MV to LV outflow tract echogenic strand was present in 76% of the patients and was increasingly prevalent with more severe EFE (52% at grade 1, 88% at grade 2, and 100% at grade 3). Compared to patients with grade 1 EFE, those with grade 2 or 3 were more likely to have a MV line (odds ratio 12.2, 95% confidence interval 3.1 to 47.9; p <0.001). Patients with grade 1 EFE were more likely to have an LV/right ventricle length ratio ≥1 (74%) than were patients with grade 2 or 3 EFE (43%) (odds ratio 3.8, 95% confidence interval 1.4 to 10.5, p = 0.009). Patients with grade 1 EFE (75%) were also more likely than patients with EFE grade 2 (34%) or 3 (11%) to have “high LV pressure” (maximum AS or mitral regurgitant jet gradient ≥20 mmHg) 2 (grade 1 relative to combined grade 2 and 3; odds ratio 2.5, 95% confidence interval 1.4 to 4.2, p <0.001).
| Variable | κ or Weighted κ | Standard Error |
|---|---|---|
| Intraobserver variability | ||
| Endocardial fibroelastosis severity grade (0–3) | ||
| Observer 1 | 0.43 | 0.08 |
| Observer 2 | 0.76 | 0.06 |
| Presence of severe endocardial fibroelastosis (yes vs no) | ||
| Observer 1 | 0.45 | 0.12 |
| Observer 2 | 0.74 | 0.10 |
| Septal endocardial fibroelastosis severity (0–2) | ||
| Observer 1 | 0.49 | 0.10 |
| Observer 2 | 0.57 | 0.09 |
| Septal endocardial fibroelastosis more prominent than elsewhere (yes vs no) | ||
| Observer 1 | 0.43 | 0.10 |
| Observer 2 | 0.57 | 0.09 |
| Mitral valve-left ventricular outflow echogenic strand (no) | ||
| Observer 1 | 0.44 | 0.13 |
| Observer 2 | 0.56 | 0.10 |
| Interobserver variability | ||
| Endocardial fibroelastosis severity grade (0–3) | 0.54 | 0.08 |
| Presence of severe endocardial fibroelastosis (yes vs no) | 0.53 | 0.13 |
| Septal endocardial fibroelastosis severity (0–2) | 0.44 | 0.10 |
| Septal endocardial fibroelastosis more prominent than elsewhere (yes vs no) | 0.43 | 0.10 |
| Mitral valve-left ventricular outflow echogenic strand (no) | 0.10 | 0.11 |
When EFE severity was collapsed into a dichotomous variable, patients with mild EFE (grade 1) had significantly greater maximum instantaneous AS gradients, larger MV annulus Z scores, and less globular LV geometry than patients with grade 2 or 3 EFE on the preintervention echocardiogram ( Table 2 ). Also, a trend was seen toward greater preintervention LV ejection fraction among fetuses with mild EFE ( Table 2 ). EFE severity was not significantly associated with the Z scores of the aortic valve or LV dimensions, any other left heart physiologic features, or right ventricular output. Among the fetuses that underwent a technically successful intervention, 74 ± 19 days elapsed between the preintervention echocardiogram and the last prenatal follow-up echocardiogram. During that period, Z scores decreased for all LV anatomic parameters: LV long axis, −0.26 ± 0.18/wk; LV short axis, −0.39 ± 0.32/wk; and LV end-diastolic volume, −0.38 ± 0.35/wk. Rates of change for these left heart Z scores did not differ according to the severity of EFE (all p >0.05). However, the rates of change in absolute (not Z score) LV short-axis and long-axis dimensions were greater in fetuses with mild (grade 1) EFE compared to those with grade 2 or 3 EFE to a degree that approached significance, and the rate of change in the LV end-diastolic volume was significantly greater in fetuses with grade 1 EFE ( Table 3 ).
| Variable | Grade 1 EFE | Grade 2 or 3 EFE | p Value |
|---|---|---|---|
| Gestational age (weeks) | 24.6 ± 2.8 | 23.8 ± 2.5 | 0.21 |
| Aortic annulus diameter Z score | −2.54 ± 0.89 | −2.47 ± 1.0 | 0.74 |
| Aortic stenosis maximum instantaneous gradient (mm Hg) | 29.7 ± 19.3 | 14.1 ± 7.7 | 0.004 |
| Left ventricular long-axis dimension Z score | 0.91 ± 1.88 | 0.18 ± 1.90 | 0.13 |
| Left ventricular short-axis dimension Z score | 2.29 ± 2.37 | 3.29 ± 2.67 | 0.12 |
| Left ventricular end-diastolic volume Z score | 1.89 ± 2.59 | 2.15 ± 4.52 | 0.78 |
| Left ventricular sphericity ⁎ | 0.63 ± 0.13 | 0.75 ± −0.11 | <0.001 |
| Left ventricular ejection fraction (%) | 24.8 ± 12.8 | 19.3 ± 9.3 | 0.059 |
| Mitral valve annulus diameter Z score | −0.83 ± 1.23 | −1.75 ± 1.08 | 0.003 |
| Mitral inflow duration Z score | −3.10 ± −1.47 | −2.70 ± 1.84 | 0.41 |
| Right ventricular long-axis dimension Z score | 1.18 ± 1.36 | 1.45 ± 1.48 | 0.46 |
⁎ Left ventricular sphericity calculated as ratio of short-axis dimension to long-axis dimension.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


