Left atrial appendage closure with the WATCHMAN device is an alternative to anticoagulation for stroke prevention in selected patients with atrial fibrillation (AF). LA device-related thrombus (DRT) is poorly defined and understood. We aimed to (1) develop consensus echocardiographic diagnostic criteria for DRT; (2) estimate the incidence of DRT; and (3) determine clinical event rates in patients with DRT. In phase 1 (training), a training manual was developed and reviewed by 3 echocardiographers with left atrial appendage closure device experience. All available transesophageal (TEE) studies in the WATCHMAN left atrial appendage system for embolic protection in patients with atrial fibrillation (PROTECT-AF) trial patients with suspected DRT were reviewed in 2 subsequent phases. In phase 2 (primary blind read), each reviewer independently scored each study for DRT, and final echo criteria were developed. Unanimously scored studies were considered adjudicated, whereas all others were reevaluated by all reviewers in phase 3 (group adjudication read). DRT was suspected in 35 of 485 patients by the site investigator, the echocardiography core laboratory, or both; 93 of the individual TEE studies were available for review. In phase 2, 3 readers agreed on 67 (72%) of time points. Based on phases 1 and 2, 5 DRT criteria were developed. In phase 3, studies without agreement in phase 2 were adjudicated using these criteria. Overall, at least 1 TEE was DRT positive in 27 (5.7%) PROTECT-AF patients. Stroke, peripheral embolism, or cardiac/unexplained death occurred in subjects with DRT at a rate of 3.4 per 100 patient-years follow-up. In conclusion, DRT were identified on at least 1 TEE in 27 PROTECT-AF patients, indicating a DRT incidence of 5.7%. Primary efficacy events in patients with DRT occurred at a rate of 3.4 per 100 patient-years follow-up, intermediate in frequency between event rates previously reported for the overall device and warfarin arms in PROTECT-AF.
The WATCHMAN left atrial appendage (LAA) closure system (Boston Scientific Corporation, Saint Paul, Minnesota) was developed as an alternative to chronic oral anticoagulation for prevention of cardioembolic stroke and peripheral embolization in patients with nonvalvular atrial fibrillation (AF). In a pivotal clinical trial (PROTECT-AF), the WATCHMAN device demonstrated superiority to warfarin for the combined end point of stroke, systemic embolism, and cardiovascular death after 3.8 years of follow-up and was recently approved by the US Food and Drug Administration. Infrequently, device-related thrombus (DRT) has been suspected on the LA surface of LAA closure systems. Although recommendations for the use of echocardiography in clinical trials and guidelines for echocardiographic core laboratory operations have been published, specific criteria for DRT identification are not available nor is the true incidence or clinical significance of DRT known. We aimed to develop consensus diagnostic criteria for DRT and apply them in an unbiased adjudication process to estimate the incidence of DRT in the PROTECT-AF trial.
Methods
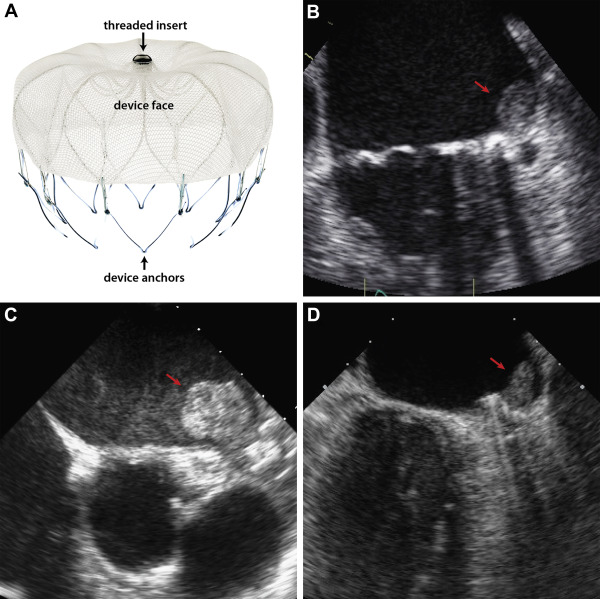
The design and results of the PROTECT-AF study have been previously published. Briefly, patients with nonvalvular AF eligible to receive warfarin for chronic thromboembolic prophylaxis were assigned in a 2:1 randomization scheme to undergo WATCHMAN device implantation or chronic warfarin therapy. The WATCHMAN device was delivered and deployed under transesophageal (TEE) and fluoroscopic guidance using a transseptal approach. The device is available in 21 to 33 mm sizes and consists of a nitinol frame with a microporous fabric cover (see Figure 1 ). A total of 485 patients (including randomized and “roll-in patients”) underwent device implantation in the PROTECT-AF trial. By study design, all patients underwent TEE at 5 time points: baseline, periprocedurally, and at 45 days, 6 months, and 12 months after implant. All TEEs were performed using multiplane imaging. Zoom imaging of the appendage was frequently used.
After 45 days, device position and peridevice flow were assessed through TEE, and patients with jet width <5 mm were eligible to discontinue warfarin at physician discretion. The patient remained on dual antiplatelet therapy (aspirin 81 to 325 mg and clopidogrel 75 mg) until the 6-month follow-up visit, at which time clopidogrel was discontinued if a repeat TEE showed no thrombus.
Our study evaluated all available follow-up TEEs in PROTECT-AF device patients in whom the study site investigator and/or the echocardiography core laboratory, suspected DRT on at least follow-up examination. Echocardiographic assessment of these studies consisted of 3 phases. In phase 1 (training), a training manual was developed using published experience with LAA closure device DRT, and pathologic studies of normal and abnormal device incorporation after implant were reviewed by 3 echocardiographers (PSD, DF, and MLM). All 3 readers are highly experienced echocardiographers with extensive previous and ongoing work imaging this LAA closure device. All 3 are level 3–trained echocardiographers.
A group review of training images (with and without thrombus) was then conducted. In phase 2 (Primary Blind Read), each reviewer independently scored each of the studies as positive or negative for DRT and developed final echo criteria. Readers also scored each DRT as laminar (basal length greater than height) or pedunculated (height greater than basal length) and noted whether the DRT obscured the device central “threaded insert” (the threaded connector on the device for the guide catheter/delivery system). Unanimously scored studies were considered adjudicated. All others were reevaluated by all 3 reviewers in phase 3 (Group Adjudication Read), using 5 prospectively developed criteria from the previous 2 phases. These included an echo density on the LA aspect of the device (1) not explained by imaging artifact; (2) inconsistency with normal healing/device incorporation; (3) visible in multiple TEE planes, (4) in contact with the WATCHMAN device; and (5) exhibiting independent motion.
For each of the cases in question, one reader selected several full-motion images which best demonstrated the suspected DRT and presented these to the group. Each case ultimately achieved consensus on the presence or absence of DRT. After adjudication, one reader (MLM) selected the image and frame in which DRT was best visualized on each study and measured the DRT area in a single imaging plane using electronic calipers. A second reader (PSD) performed interobserver measurements. All data were entered into a 21 code of federal regulations part 11 compliant database with an audit trail, electronic signatures for locking data, and secure password protection. Clinical and demographic variables were subsequently compared for subjects with DRT versus all other device patients in PROTECT-AF. For subjects with DRT, primary efficacy event rates (ischemic stroke, peripheral embolism, and cardiovascular/unexplained death) were calculated.
Demographic data are described as mean ± SD for continuous variables and number (percentage) for categorical variables and compared using the Student t tests and chi-square tests, respectively. Intraclass correlation was calculated for the thrombus length, width, and area measurement between 2 readers. Kappa coefficients were calculated for categorical variables used to define thrombus by 2 readers. There were no adjustments for multiple comparisons of multiple observations within subjects. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina).
Results
Our study evaluated 93 follow-up TEEs in 35 PROTECT-AF device patients who underwent WATCHMAN device implantation in which the study site investigator and/or echocardiography core laboratory suspected DRT (33 at 45-day follow-up, 33 at 6-month follow-up, and 27 at 1-year follow-up). In phase 2, the 3 readers agreed on 67 (72%) of time points (DRT n = 23, no DRT, n = 44; Figure 2 ). DRT was adjudicated as present in 15 of 26 TEEs (58%) evaluated in phase 3. Of the 15 studies interpreted as DRT positive by 1 reviewer in phase 2, 9 were adjudicated as DRT positive in phase 3. Of the 11 studies interpreted as DRT positive by 2 reviewers in phase 2, 6 were adjudicated as DRT positive in phase 3 (see Figure 2 ). DRT criteria scores were higher in phase 3 cases adjudicated as positive for DRT, than in those with no DRT (4.4 vs 0.9, maximum score 5, with 1 point assigned for each positive criteria, p <0.001; see Table 1 ). Overall, at least 1 TEE was DRT positive in 27 PROTECT-AF patients (see Table 2 ). Of the 5 DRT criteria used in phase 3, exhibition of independent motion was least commonly present in DRT positive studies (8 of 15).
| Study number | Final Read | Criteria for thrombus present/absent | |||||
|---|---|---|---|---|---|---|---|
| Thrombus +/0 | Discrete echo density not artifact | Inconsistent w nl healing | Visible inmultiple planes | In contact with device | Independent motion | Total Score | |
| 1 | + | 1 | 1 | 0 | 1 | 1 | 4 |
| 2 | + | 1 | 1 | 1 | 1 | 1 | 5 |
| 3 | + | 1 | 1 | 1 | 1 | 1 | 5 |
| 4 | + | 1 | 1 | 1 | 1 | 0 | 4 |
| 5 | + | 1 | 1 | 1 | 1 | 0 | 4 |
| 6 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 7 | + | 1 | 1 | 1 | 1 | 0 | 4 |
| 8 | + | 1 | 1 | 1 | 1 | 0 | 4 |
| 9 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| 10 | + | 1 | 1 | 1 | 1 | 0 | 4 |
| 11 | + | 1 | 1 | 1 | 1 | 1 | 5 |
| 12 | + | 1 | 1 | 1 | 1 | 0 | 4 |
| 13 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | + | 1 | 1 | 1 | 1 | 1 | 5 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| 19 | 0 | 1 | 0 | 1 | 0 | 1 | 3 |
| 20 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 21 | + | 1 | 1 | 1 | 1 | 0 | 4 |
| 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 23 | + | 1 | 1 | 1 | 1 | 1 | 5 |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | + | 1 | 1 | 1 | 0 | 1 | 4 |
| 26 | + | 1 | 1 | 1 | 1 | 1 | 5 |
| MONTHS | |||||
|---|---|---|---|---|---|
| Age | Gender | CHADS 2 Score | 1.5 | 6 | 12 |
| 63 | Man | 1 | ▪ | □ | ▪ |
| 65 | Man | 1 | □ | □ | □ |
| 66 | Man | 1 | ▪ | ▪ | ? |
| 66 | Man | 1 | □ | □ | □ |
| 67 | Man | 2 | □ | ▪ | ? |
| 68 | Man | 2 | □ | ? | ? |
| 71 | Man | 1 | □ | ▪ | □ |
| 71 | Man | 4 | □ | □ | ▪ |
| 72 | Woman | 2 | □ | ▪ | ▪ |
| 72 | Man | 3 | □ | ▪ | □ |
| 72 | Woman | 1 | □ | ▪ | ? |
| 73 | Man | 1 | ▪ | □ | □ |
| 74 | Man | 1 | ? | ▪ | ▪ |
| 74 | Man | 3 | □ | ▪ | ? |
| 75 | Man | 3 | ▪ | ▪ | □ |
| 75 | Man | 3 | ▪ | ▪ | ? |
| 76 | Man | 2 | □ | ? | ▪ |
| 76 | Man | 3 | □ | □ | □ |
| 77 | Woman | 1 | □ | □ | □ |
| 77 | Man | 2 | □ | ▪ | ▪ |
| 77 | Man | 2 | □ | □ | □ |
| 77 | Man | 1 | □ | ▪ | □ |
| 78 | Man | 2 | □ | □ | ▪ |
| 78 | Woman | 5 | □ | □ | □ |
| 79 | Man | 2 | □ | □ | ▪ |
| 79 | Man | 4 | □ | ▪ | □ |
| 81 | Man | 4 | □ | □ | ▪ |
| 82 | Woman | 4 | □ | ▪ | □ |
| 83 | Man | 5 | □ | □ | ? |
| 83 | Man | 2 | □ | ▪ | □ |
| 84 | Man | 4 | □ | ▪ | ▪ |
| 84 | Man | 2 | □ | ▪ | ▪ |
| 86 | Woman | 3 | ▪ | □ | ? |
| 86 | Woman | 5 | □ | ▪ | ▪ |
| 87 | Man | 3 | ▪ | ▪ | □ |
Incident DRT was present at 45 days in 7 patients, with higher prevalence at 6 and 12 months (19 and 12 patients, respectively [see Figures 1 and 3 ]). Of the 11 patients with DRT at 2 time points, 4 had DRT identified at 45 days and 6 months, 6 patients had DRT identified on both the 6- and 12-month study, and 1 patient had DRT identified on the 45-day and 12-month study (6-month study with negative findings). In 5 patients, DRT was initially recognized on the 12-months follow-up study. No patient had DRT identified on all 3 follow-up studies.
DRT were described as laminar in 27 cases and pedunculated in 11 cases. In 35 of 38 cases, the DRT covered the device “threaded insert.” Mean DRT area was 1.5 ± 2.0 cm 2 , and median DRT area was 1.0 cm 2 (interquartile range 0.6 to 1.7 cm 2 ). Examples of normal device incorporation and DRT are shown in Figures 1 and 3 , and Videos 1 to 3 . Intraclass correlation was 0.96 for area, whereas κ statistic was 0.53 for location and 0.62 for description.
Baseline clinical and demographic characteristics of patients with DRT are presented in Table 3 ; those with DRT were older and more commonly had permanent AF versus those without DRT in the PROTECT-AF trial. Primary efficacy events for patients with DRT are presented in Table 4 , occurring at a rate of 3.4 per 100 patient-years (95% CI 1.4 to 6.7 per 100 patient-years).
| DEVICE RELATED THROMBUS | ||||
|---|---|---|---|---|
| Variable | Total (n = 485) | Yes (n = 27) | No (n = 458) | P-Value |
| Age (years ± SD) | 71.8 ± 8.7 | 76.4 ± 6.3 | 71.6 ± 8.8 | 0.004 |
| Age > 75 years | 201 (41.4%) | 16 (59%) | 185 (40.4%) | 0.053 |
| Gender | 0.191 | |||
| Male | 341 (70.3%) | 22 (82%) | 319 (69.7%) | |
| Female | 144 (29.7%) | 5 (19%) | 139 (30.3%) | |
| Race | 0.827 | |||
| Asian | 2 (0.4%) | 0 | 2 (0.4%) | |
| Black/African American | 8 (1.6%) | 0 | 8 (1.7%) | |
| Caucasian | 447 (92.2%) | 25 (93%) | 422 (92.1%) | |
| Hispanic / Latino | 26 (5.4%) | 2 (7%) | 24 (5.2%) | |
| Other | 2 (0.4%) | 0 | 2 (0.4%) | |
| Atrial Fibrillation Pattern | 0.005 | |||
| Unknown | 5 (1.0%) | 0 | 5 (1.1%) | |
| Paroxysmal | 215 (44.3%) | 5 (19%) | 210 (45.9%) | |
| Persistent | 97 (20.0%) | 4 (15%) | 93 (20.3%) | |
| Permanent | 168 (34.6%) | 18 (67%) | 150 (32.8%) | |
| Prior Heart Failure | 121 (24.9%) | 9 (33%) | 112 (24.5%) | 0.300 |
| Prior TIA/Stroke | 88 (18.1%) | 5 (19%) | 83 (18.1%) | 1.000 |
| History of Hypertension | 433 (89.3%) | 24 (89%) | 409 (89.3%) | 1.000 |
| Diabetes Mellitus | 120 (24.7%) | 7 (26%) | 113 (24.7%) | 0.883 |
| CHADS2 | ||||
| 1 | 160 (33.0%) | 7 (26%) | 153 (33.4%) | |
| 2 | 170 (35.1%) | 8 (30%) | 162 (35.4%) | |
| 3 | 94 (19.4%) | 6 (22%) | 88 (19.2%) | 0.404 |
| 4 | 39 (8.0%) | 5 (19%) | 34 (7.4%) | |
| 5 | 19 (3.9%) | 1 (4%) | 18 (3.9%) | |
| 6 | 3 (0.6%) | 0 | 3 (0.7%) | |
| CHADS2 > 3 | 61 (12.6%) | 6 (22%) | 55 (12.0%) | 0.132 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


