Fig. 2.1
Normal mitral valve anatomy in a neonate. (a) Mitral valve. The posteromedial papillary muscle usually consists of multiple papillary muscle bundles. On the other hand, the anterolateral papillary muscle usually consists of a single muscle bundle. Note the prominent strut chordae from the top of each papillary muscle inserting on the rough zone of the anterior leaflet. Asterisks indicate the left and right fibrous trigone. (b) Fibrous continuity of the mitral and aortic valve. Arrows indicate fibrous continuity of the anterior mitral leaflet and aortic valve. AL anterior leaflet, ALPM anterolateral papillary muscle, L left coronary cusp, LA left atrium, LT left trigone, LVOT left ventricular outflow tract, N noncoronary cusp, PFO patent foramen ovale, PL posterior leaflet, PMPM posteromedial papillary muscle, R right coronary cusp, S strut chordae
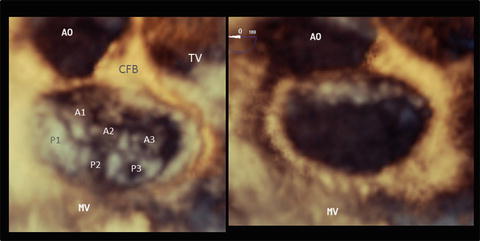
Fig. 2.2
An en face image of a normal mitral valve by three-dimensional echocardiography. The left panel shows a closed mitral valve and the right panel shows an opened valve image. The three scallops of the posterior leaflet are referred to as the anterolateral (P1), middle (P2), and posteromedial (P3) sections. The corresponding sections of the anterior leaflet are labeled A1, A2, and A3, respectively. AO aorta, CFB central fibrous body, MV mitral valve, TV tricuspid valve
The chordae tendineae are classified into three groups: (1) first-order chordae (also called marginal/free-edge chordae), which insert on the free edge of the leaflet, (2) second-order chordae (also called rough zone chordae) that insert on the ventricular surface of the leaflet beyond the free edge, forming the rough zone of the leaflet, and (3) third-order chordae (also called basal chordae), which are unique to the posterior leaflet and arise directly from the ventricular wall or from trabeculations and insert on the basal zone of the posterior leaflet. The two distinctive thick and strong second-order chordae of the anterior leaflet are called strut chordae and arise from the tip of the papillary muscle and insert on the rough zones (Fig. 2.1). Because of their distinctive morphology of chordal branching that resembles the ribs of a fan, the chordae that insert into the commissure are called fan-shaped chordae (also called commissural chordae).
Two groups of papillary muscles are located beneath the commissures, occupying anterolateral and posteromedial positions. The anterolateral papillary muscle is usually a single muscle bundle, while the posteromedial papillary muscle consists of two or three papillary muscle bundles (Fig. 2.1). Both papillary muscles usually have separate heads and the number and shape of the papillary muscle bundles vary among individuals.
2.1.3 Normal Anatomy of the Tricuspid Valve
Figure 2.3 shows the normal tricuspid valve (TV) of a neonate, the same specimen shown in Fig. 2.1. Although there are many variations to the normal morphology of a TV, the TV is generally accepted to consist of three leaflets: the anterior, septal, and posterior leaflets. Figure 2.4 shows an en face image of a normal TV constructed by transesophageal 3DE. The leaflet and chordae are thinner than those of the MV. The anterior leaflet (also called the superior leaflet) is the largest of the three leaflets, is located in the anterosuperior position, and guards the orifice of the right ventricular outflow tract. The septal leaflet (also called the medial leaflet) is usually larger than the posterior leaflet. Although most of the septal leaflet’s basal attachment is to the interventricular septum, its attachment sometimes extends to the inferior wall. A small fold is frequently observed at the transition between the septal and posterior leaflets. The septal leaflet has a chordal attachment to the ventricular septum, which limits its mobility. These distinctive anatomical features of the sepal leaflet allow the TV to be distinguished from the MV. The posterior leaflet (also called the inferior leaflet) is the smallest leaflet and is located at the inferior position. Compared to the MV, the leaflet morphology of the TV is highly variable, with many indentations of variable depth. The annulus of the TV usually lacks a solid ringlike fibrous cord and is pathologically just a continuation of the fibrous tissue of the leaflet to the subendocardial fiber. The annulus of the septal leaflet is especially indistinct because the anterior part of the leaflet merges with the membranous interventricular septum and is apically displaced from the atrioventricular junction. The commissure between the anterior and septal leaflets (anteroseptal commissure) is located at the most cranial position of the membranous septum and the fan-shaped chordae from the medial papillary muscle attach to the septal and anterior leaflets. The medial papillary muscle is on the bifurcation of the anterior and posterior limbs of the trabecula septomarginalis or is sometimes absent, in which case the chordae arise directly from the trabecula septomarginalis or the crista supraventricularis. The commissure between the anterior and posterior leaflets (anteroposterior commissure) is located roughly at the acute margin of the right ventricle, and beneath it is an anterior papillary muscle, which is the largest papillary muscle, in the right ventricle and has a moderator band attached to its base. The commissure between the posterior and septal leaflets (posteroseptal commissure) is located at the junction of the inferior and septal walls, and beneath it is a posterior papillary muscle. The posterior papillary muscle is on the inferior wall and at the most medial position. Its size varies considerably and is usually small (Fig. 2.3) [5].
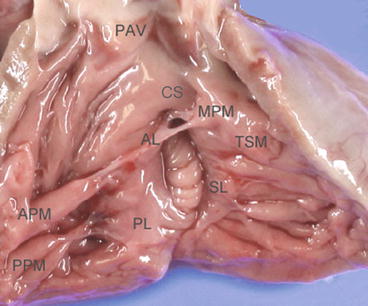
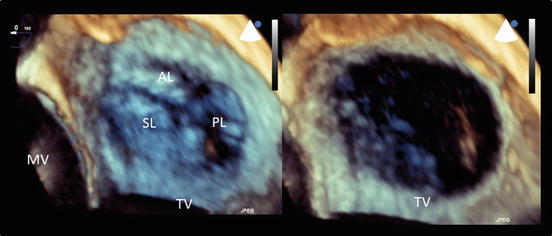

Fig. 2.3
Normal tricuspid valve anatomy in a neonate. The tricuspid valve consists of three leaflets. The septal leaflet is tethered by chordae from the ventricular septum and is less mobile compared to the other two leaflets. AL anterior leaflet, APM anterior papillary muscle, CS crista supraventricularis, MPM medial papillary muscle, PAV pulmonary artery valve, PL posterior leaflet, PPM posterior papillary muscle, SL septal leaflet, TSM trabecula septomarginalis

Fig. 2.4
An en face image of a normal tricuspid valve obtained using three-dimensional echocardiography. The left panel shows a closed tricuspid valve and the right panel shows an opened valve image. The tricuspid valve consists of three leaflets. AL anterior leaflet, MV mitral valve, PL posterior leaflet, SL septal leaflet, TV tricuspid valve
2.2 Anomalies of the Atrioventricular Valve
The etiologies of AVV disease are as follows: congenital, degenerative, inflammatory, endocarditis, rheumatic, ischemic, cardiomyopathies, traumatic, and iatrogenic. These etiologies are associated with the anatomical abnormality or malfunction of one or multiple components constituting the AVV complex, thereby causing regurgitation or stenosis of the AVV. Carpentier et al. classified the mechanisms of mitral regurgitation into three categories [7, 8]. This classification is applicable to the TV or common AVV:
Type I: normal leaflet motion (annular enlargement, leaflet perforation, or cleft)
Type II: excessive leaflet motion (flail leaflet, ruptured chordae, prolapse, or billowing)
Type III: restricted leaflet motion:
(a)
Short leaflet or chordae
(b)
Leaflet tethering by the papillary muscle
In congenital AVV disease, the multiple pathologies outlined above usually coincide and cause regurgitation and/or stenosis.
2.2.1 Mitral Valve Disease
The classification of congenital mitral valve anomalies is shown in Table 2.1 [6].
Table 2.1
Congenital mitral valve anomalies
1. Anomalies of the leaflet | Cleft mitral valve |
Double orifice mitral valve | |
Excessive (accessory) mitral valve tissue | |
Ebstein’s anomaly | |
Hypoplasia/dysplasia/atresia of leaflet | |
2. Anomalies of the commissure | Commissural fusion |
3. Anomalies of the chordae tendineae | Short chordae |
Chordal elongation | |
Chordal rupture | |
4. Anomalies of the papillary muscle | Parachute mitral valve |
Mitral arcade/hammock mitral valve | |
Obstruction by abnormal papillary muscle | |
5. The supramitral ring | Supramitral ring/membrane |
6. Combination of the above anomalies |
1.
Isolated mitral valve cleft (Fig. 2.5): Figure 2.5 shows en face images of an isolated MV cleft by transesophageal 3DE. This case was not associated with an atrioventricular septal defect and the regurgitation was from a cleft. This anomaly was first reported by Petitalot in 1987 [9], and the cleft is often oriented towards the left ventricular outflow tract rather than the ventricular septum, as is usually seen in atrioventricular septal defects. However, in this particular patient, the cleft points towards the ventricular septum.
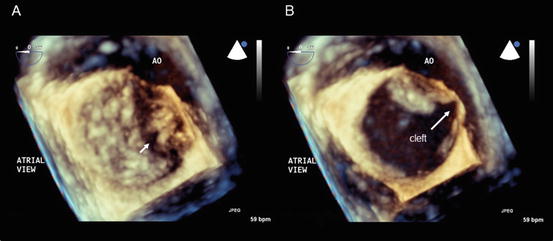

Fig. 2.5
Isolated mitral valve cleft. En face images of a mitral valve at the closed (a) and opened (b) positions obtained using transesophageal three-dimensional echocardiography. The arrow indicates the mitral valve cleft. AO aorta
2.
Double orifice mitral valve (Fig. 2.6): A double orifice MV was first described by Greenfield in 1876 and is a rare AVV anomaly characterized by the presence of two or more orifices in the AVV leaflet, each having an independent chordal attachment to the papillary muscles [10]. A double orifice MV rarely occurs as an isolated form and is usually associated with an atrioventricular septal defect, ventricular septal defect, truncus arteriosus, pulmonary stenosis, coarctation or interruption of the aortic arch, a bicuspid aortic valve, tetralogy of Fallot, or Ebstein’s anomaly. A partial atrioventricular septal defect is most commonly seen, accounting for 41 % of associated anomalous lesions. Mitral regurgitation is the most common functional abnormality (43 % of patients), followed by mitral stenosis (13 %) and their combination (7 %). Of note, no functional abnormality of the MV is observed in 37 % of patients [11].
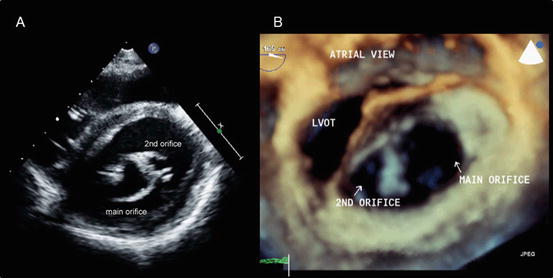

Fig. 2.6
Double orifice mitral valve. (a) A transthoracic two-dimensional echocardiography image. (b) An en face image obtained using transesophageal three-dimensional echocardiography. LVOT left ventricular outflow tract
3.
Rheumatic mitral valve disease (Fig. 2.7): Figure 2.7 shows a transthoracic 3DE image of a 12-year-old Afghan boy suffering from rheumatic fever with commissural fusion causing significant MV stenosis. The rheumatic process causes leaflet thickening and fusion of commissures, resulting in limited leaflet movement and a narrowing of the mitral orifice. The chordae tendineae are also involved in fusion, shortening, fibrosis, and calcification, leading to restricted leaflet movement, leaflet malcoaptation, and regurgitation [12].
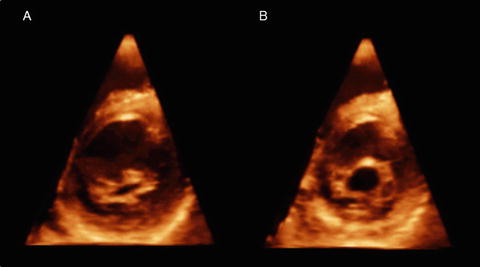

Fig. 2.7
Rheumatic mitral valve disease. Transthoracic three-dimensional images of a mitral valve at the closed (a) and opened (b) positions. Note significant commissural fusion (b)
2.2.2 Tricuspid Valve Disease
TV anomalies are shown in Table 2.2 [13].
Table 2.2
Tricuspid valve anomalies
1. Congenial | Ebstein’s anomaly |
Tricuspid valve dysplasia | |
Tricuspid valve hypoplasia/atresia | |
Tricuspid valve cleft | |
Double orifice tricuspid valve | |
Unguarded tricuspid valve orifice | |
Straddling of chordae tendineae | |
2. Right ventricular disease | Arrhythmogenic right ventricular cardiomyopathy |
Uhl’s disease | |
Endocardial fibroelastosis | |
Increased right ventricular pressure | |
3. Acquired | Annular dilation |
Left-sided valvular heart disease | |
Endocarditis | |
Trauma | |
Carcinoid heart disease | |
Rheumatic heart disease | |
Tricuspid valve prolapse | |
Iatrogenic (radiation, drugs, biopsy, pacemaker, ICD) | |
4. Right ventricular dilation | Pulmonary hypertension |
Atrial septal defect | |
Anomalous pulmonary venous return | |
Pulmonary valve insufficiency |
The functional abnormality of TV disease most commonly presents as tricuspid regurgitation. Isolated TV stenosis is very rare and is observed in countries where rheumatic heart disease is prevalent. Rather, TV stenosis commonly presents as combined lesions of stenosis and regurgitation.
The most common causes of congenital tricuspid regurgitation are Ebstein’s anomaly and TV dysplasia. These two entities are clinically similar but anatomically different. Ebstein’s anomaly is characterized by an inferior displacement of the proximal hinge point of the septal and posterior leaflets from the atrioventricular junction and the existence of an atrialized ventricle (Fig. 2.8a,b). On the other hand, the basal attachment of the TV is normal in TV dysplasia. Aaron described the characteristic features of TV dysplasia as follows: [1] focal or diffuse thickening of the leaflets; [2] deficient development of the chordae tendineae and papillary muscles, most often binding down or tethering the valve margin; [3] improper separation of valve components from the ventricular wall; and [4] focal agenesis of valvular tissue [14].
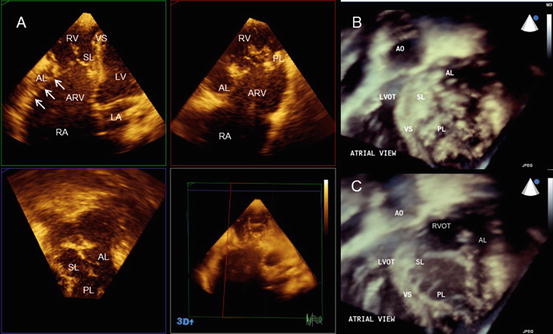

Fig. 2.8
Ebstein’s anomaly. (a) The simultaneous orthogonal 3 planes of a tricuspid valve. Note the significant plastering of the septal and posterior leaflets. Arrows indicate the hyphenated distal attachment of the anterior leaflet to the right ventricular free wall. (b) An en face image of a tricuspid valve in the closed position. (c) An en face image of a tricuspid valve in the opened position. AL anterior leaflet, AO aorta, ARV atrialized right ventricle, LA left atrium, LV left ventricle, LVOT left ventricular outflow tract, PL posterior leaflet, RA right atrium, RV right ventricle, RVOT right ventricular outflow tract, VS ventricular septum
1.




Ebstein’s anomaly (Fig. 2.8): The crucial feature of Ebstein’s anomaly is the rotational displacement of the hinge point of the TV leaflet, with maximal apical displacement occurring at the junction of the septal and posterior leaflets and no displacement of the anterior leaflet. This apical displacement creates the atrialized portion of the basal right ventricle. The anterior leaflet is usually large, with normal annular attachments at the atrioventricular junction. However, it is commonly associated with restricted motion. The restriction of the anterior leaflet is caused by short chordae and the expansion of the anterior papillary muscle onto the ventricular surface. In its severe form the chordae tendineae are absent, and linear or hyphenated distal attachment of the leaflet edge to the ventricular wall is observed [15, 16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


