Clinical presentations of pulmonary aspergillosis. AF Aspergillus fumigatus, PDC chronic pulmonary disease, CF cystic fibrosis, ABPA allergic pulmonary aspergillosis
Invasive Pulmonary Aspergillosis
The greatest risk factors for invasive pulmonary aspergillosis (IPA) in the pediatric population are the presence of neutropenia, chronic steroids therapy, immunosuppression therapy, neoplastic disease, and acquired immunodeficiency. Use of chemotherapy and immunosuppressive agents has increased the rate of aspergillosis during the past decades. In a study examining the autopsies of immunocompromised patients between 1978 and 1992, the rate for invasive aspergillosis varied between 0.4% and 3%, with a rate increase between 17% and 60% in the autopsies performed during the past years. The mortality rate exceeds 50% for neutropenic patients, and it may reach 90% in receptors of bone marrow transplant.
Aspergillus enters the lower respiratory tract through spore inhalation, and less frequently, the infection can initiate in other locations, such as the paranasal sinus, digestive tract, or the skin. The most frequent presentation forms of IPA include pulmonary and upper airway disease, both with a high mortality. Clinically, its presentation is nonspecific: febrile syndrome, productive cough, and dyspnea. Pleura pain may be present, as well as hemoptysis, associated with vascular microthrombosis and small areas of pulmonary infarction. IPA is one of the most common causes of hemoptysis in neutropenic patients, and it may be associated with cavity appearance during neutrophil recovery. From the radiological aspect, early findings are also nonspecific, with rounded opacities tending to appear, besides pleural base infiltrations that may suggest lung necrosis and cavitated lesions.
Hematogenic dissemination to other organs is possible, fundamentally to the central nervous system, developing meningitis, epidural abscess, brain stroke, etc.
A significantly lower percentage of patients have presented tracheobronchitis caused by Aspergillus, which is the isolated invasion of the tracheobronchial tract. Three presentations have been described for this disease: obstructive, pseudomembranous, and ulcerative.
The diagnosis of IPA i s based on the presence of risk factors, signs, symptomatology, chest X-ray findings, histopathological findings, and the development of Aspergillus in the culture.
Its diagnosis is difficult in immunocompromised patients. It is necessary to keep a high suspicion index when working with higher-risk groups. Definitive diagnosis is based on the histopathological identification of hyphae invading the lung tissue, along with a positive culture for Aspergillus in the same site. Histopathological findings and the type of local antiinflammatory reaction will depend on the immunological condition of the host.
The steps to follow after Aspergillus has been isolated in the sputum will depend on the condition of the host. Generally, immunocompetent patients will present a colonization with no clinical consequences, and thus antifungal therapy would not be indicated, but IPA should be ruled out. In contrast, when Aspergillus has been isolated in immunocompromised patients, it has a high positive predictive value for this infection. Nevertheless, negative sputa do not rule out IPA.
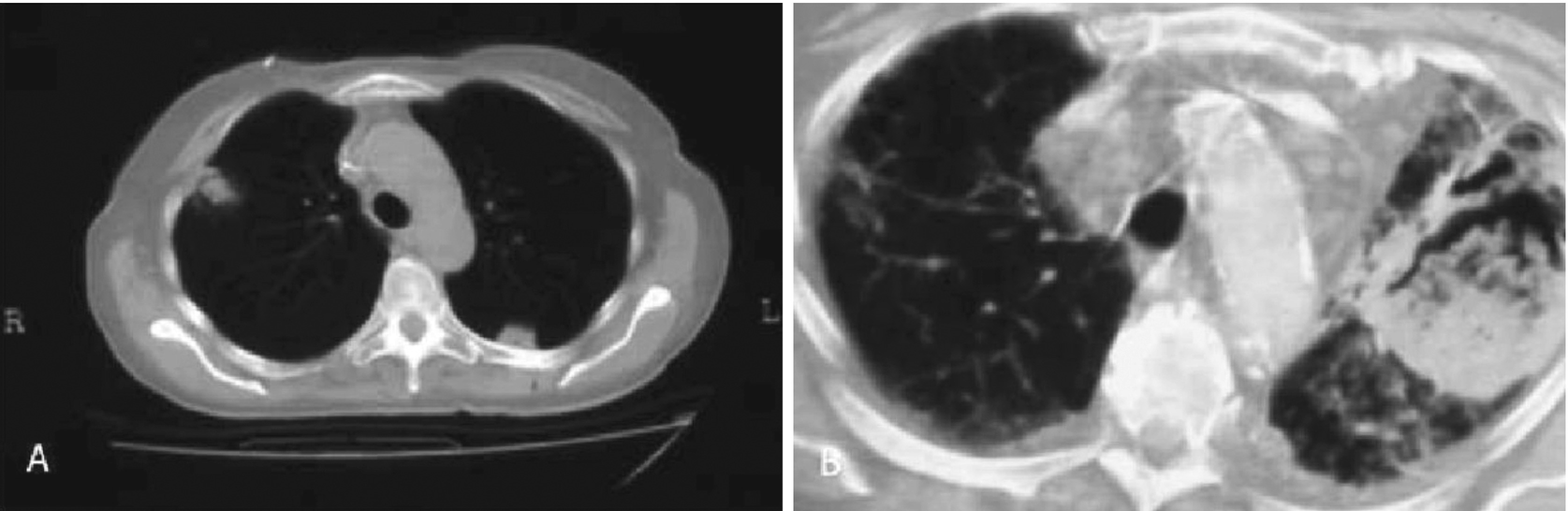
Invasive pulmonary aspergillosis. Chest computed axial tomography of an immunosuppressed teenager presenting with nodular subpleural lesions and halo sign (a) and half-moon sign with condensation (b)
Bronchoalveolar lavage (BAL) may be useful, although Aspergillus recovery percentages in the fluid obtained are very variable. It can be used to search for anti-Aspergillus antibodies and rule out other infections.
Recently, Aspergillus antigen detection techniques have been used, such as galactomannan in body fluids. This antigen can be present days before the appearance of symptoms, signs, and radiological alterations. Its main limitations are its low positive predictive power and the possibility of false positives and false negatives.
IPA is associated with high mortality rates. The treatment considers amphotericin B as first line drug, which is associated to nephrotoxicity, hydroelectric alterations, and hypersensitivity. New liposomal presentations of this drugs have less secondary effects, with usually adequate antifungal action. Voriconazole has been approved as first line drug for the treatment of IPA, and is associated to less adverse effects, thus it is more tolerable. Generally, treatments must be indicated for prolonged periods, up to a year. Caspofungin, micafungin, and anidulafungin are therapeutic options for patients with refractory IPA when the usual treatment has been used, or if the patient cannot tolerate it.
Surgical resection should be considered in the cases of massive hemoptysis, lung lesions close to vital structures (great vessels, pericardium) or residual lesions in patients who are still immunosuppressed.
Chronic Necrotizing Pulmonary Aspergillosis
Chronic necrotizing pulmonary aspergillosis is very infrequent, and there are only isolated cases or case reports for this type of presentation of the disease.
It has been mostly described in adults with alterations of local defense mechanisms associated with chronic pulmonary disease (chronic obstructive pulmonary disease, sarcoidoisis, tuberculosis or radiotherapy history) or with systemic diseases that cause mild degrees of immunosuppression (malnutrition, connective tissue diseases, chronic liver disease, alcoholism, liver failure, etc).
Clinically, it can have a certain degree of overlapping with aspergilloma, and it is characterized by the presence of general symptoms (fever, fatigue, weight loss), productive cough, and hemoptysis. Image studies evidence consolidation, pleural enlargement, and cavitary lesions, which progress slowly. Sputum cultures tend to be positive for Aspergillus. The confirmation of the diagnosis is done through the histopathological findings of hifa in lung tissue and positive culture for the fungus. The treatment consists in the administration of antifungal agents such as itraconazole or voriconazole. The surgical option should be used only for completely localized lesions in patients with good respiratory reserve or if the therapy fails.
Aspergilloma
Aspergilloma is the most common lung disease that usually takes place in a preexistent cavity of the lung. An aspergilloma, or fungal ball, is composed of the hyphae of the fungus, inflammatory cells, fibrin, mucus, and remaining tissue. Some cavitating lung diseases such as tuberculosis, sarcoidosis, bronchiectasis, bronchial cysts, and bullas may be complicated by aspergillomas. The fungal ball can move within the cavity, but it does not invade the pulmonary parenchyma or the blood.
In a study conducted on 544 patients with pulmonary cavities secondary to tuberculosis, 11% had radiological evidence of aspergilloma.
Most of the patients are asymptomatic. When there are symptoms, most patients present hemoptysis. Less frequently, cough and dyspnea related to the underlying disease may also appear. Fever is secondary to the underlying disease, or to bacterial overinfection. Poor prognosis risk factors include the seriousness of the underlying pulmonary disease, the increase in the size or the number of the lesions, immunosuppression, the increase of IgG-specific antibody titers, and hemoptysis.
Diagnosis is made considering the clinical and radiographic characteristics showing a mobile intracavity mass, located in a preexistent cavity with a peripheral half-moon space, with microbiological and serological evidence of Aspergillus spp. Computerized axial tomography (CAT) confirms the findings. Sputum cultures for Aspergillus spp. are positive only in 50% of the cases. IgG antibodies against Aspergillus are positive in most cases.
Treatment is considered only when the patients present with symptoms, usually with hemoptysis. There is no agreement about the best treatment. Administration of percutaneous amphotericin B guided by chest CT seems to be efficient, especially in patients with massive hemoptysis. There is not enough knowledge about the use of IV amphotericin B. Itraconazole has a high tissue penetration, and it may be useful in some patients, showing improvements in 50% of cases. The role of voriconazole has not been established.
Surgical removal of the cavity with exeresis of the fungal ball is indicated for patients with recurrent hemoptysis.
Allergic Bronchopulmonary Aspergillosis
This disease is caused by the hypersensitivity of AF antigens. Although this is the main pathogen causing allergic bronchopulmonary aspergillosis (ABPA), there may be other related pathogens involved. In most cases, it affects patients with asthma or cystic fibrosis. It has been estimated that 2% of asthmatic patients, and around 7–14% of asthmatic patients who are steroid dependent, have ABPA. Incidence is greater in atopic patients.
In the case of CF, it is variable in relation to different locations, but according to several authors, around 1–5% of patients with CF may develop ABPA at some point. The pathogenesis of ABPA is currently unknown. It has been proposed that inhaled spores are trapped in the mucus of the large airway. The spores may germinate when they develop hyphae, which release antigens and cause the immune response. The hypersensitivity reaction they cause is characterized by IgG and IgE production, as well as specific antibodies for AF. This reaction, combined with the production of cytotoxic metabolites, such as AF proteolytic enzymes, cause a local immunosuppression reaction, phagocytosis inhibition, and flaking of epithelium cells. As a whole, this reaction causes AF colonization and allows for its persistence in the airway, with lung infiltrations, tissue damage, and finally lung tissue destruction.
ABPA tends to be clinically suspected, and it is confirmed through serum tests and chest X-ray. Most patients present with episodic wheezing, sputum expectorations, dark blockages, dyspnea, pleural chest pain, fever, and sometimes hemoptysis that do not respond to the usual therapy.
Chest X-ray can be normal during the first stages of the disease. During exacerbations, brief central lung infiltrations appear in the superior lobes, along with atelectasis. In subsequent stages of the disease, central bronchiectasis and lung fibrosis may appear. Chest CT images can show bronchiectasis in more than three lobes, centrilobular nodules, and mucoid impaction, which all suggest this diagnosis. In CF, chest CT may show lung infiltrations and central bronchiectasis, that are not so useful as in asthma, because they are commonly found as a consequence of the disease.
Lung function tests do not yield characteristic results of ABPA, and show reversible obstructive lung disease, which becomes irreversible in the advanced stages of the disease. Oral steroids are the main treatment, which will vary according to the stage of the disease and if the patient suffers from asthma or CF.
Allergic Bronchopulmonary Aspergillosis in Asthma
Sensitization of AF antigens occurs in 28% of patients with asthma, but ABPA is found in 2% of asthma patients, 7–14% of steroid-dependent asthma patients, and 33% of severe asthma patients. Sensitization to AF antigens may cause severe obstruction of the air flow and therefore greater use of oral steroids.
Diagnosis criteria for ABPA in asthma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


