Arteriovenous Hemodialysis Access—Beyond Traditional
THOMAS S. HUBER
Presentation
A 57-year-old obese female with a history of end-stage renal disease presents to your clinic for permanent hemodialysis access. She has been on dialysis for the past 7 years and is currently dialyzing through a tunneled, cuffed catheter in her right femoral vein. Her end-stage renal disease is secondary to both diabetes and hypertension. She is right handed. She has a complex access history and has had permanent access procedures in both upper extremities including a left brachioaxillary prosthetic arteriovenous access that had to be removed secondary to infection. Most recently, she was dialyzing through a right upper extremity brachial-basilic autogenous access. She has a known axillary/subclavian vein stenosis on the right that was remediated several times with a combination of percutaneous procedures including balloon angioplasty and stenting. The right-sided central vein occlusion, likely the etiology of the most reason access failure, could not be crossed with a wire during the last remedial attempt.
Differential Diagnosis
The patient requires permanent hemodialysis access and has been labeled as “complex” given her prior access history, obesity, and known central vein occlusion. Although most access surgeons have a clinical impression of what constitutes “complex” or “tertiary” problems, the term is poorly defined, and there are no clear recommendations or guidelines from the Kidney Disease Outcome Quality Initiatives (KDOQI), the standard of care for maintaining hemodialysis access. The anatomic and patient comorbidities that complicate the construction of a permanent hemodialysis, potentially leading to the definition of a complex access, are shown in Table 1. Central vein occlusions and/or stenoses are likely the most common complex problem given the widespread use of central vein catheters for both dialysis- and non–dialysis-related indications. Unfortunately, the cohort of patients with complex access problems is likely expanding given the improvements in care for patients with end-stage renal disease, their improved life expectancies, and the reliance on central venous catheters as noted above.
TABLE 1. Anatomic and Patient Comorbidities Complicating Permanent Hemodialysis Access Procedures

Workup
The initial approach to patients with “complex” access problems is identical to that for those presenting for their first access (Fig. 1). This systematic approach can help identify potential problems or contraindications to an access (e.g., arterial inflow stenosis secondary to subclavian stenosis) and, thereby, suggest solutions or remedial treatments (e.g., subclavian angioplasty). It is based upon the KDOQI guidelines with a strong emphasis on creating autogenous accesses due to their improved patency and decreased infectious complication rates. Constructing a permanent hemodialysis access is similar to a lower extremity bypass procedures and based upon the principles of vascular surgery including adequate inflow, adequate outflow, and a suitable conduit. True complex access problems are not all that common, and it is possible to construct a traditional access in most patients, usually an autogenous access.
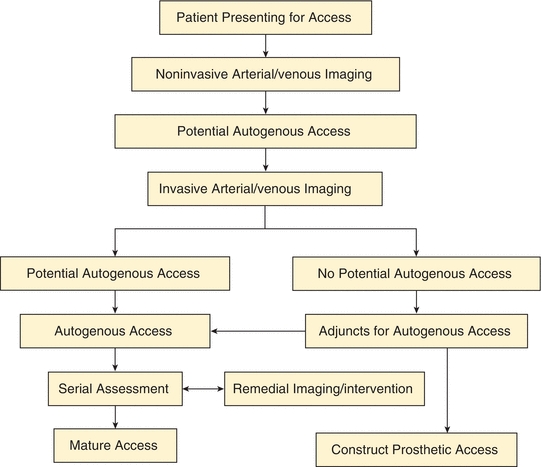
FIGURE 1 An algorithm for patients presenting for permanent hemodialysis access is shown. Note that Invasive Arterial/venous Imaging now includes both catheter- and CT-based arteriography and venography. Patients with No Potential Autogenous Access due to peripheral veins that are insufficient diameter (less than 3 mm) are either reimaged in the operating room with ultrasound after induction of anesthesia or the veins are dissected and explored directly. Adjuncts for Autogenous Access include endovascular treatment of arterial inflow/venous outflow lesions and composite access configurations. (Reproduced from Huber TS, et al. Prospective validation of an algorithm to maximize arteriovenous fistulae for chronic hemodialysis access. J Vasc Surg. 2002;36:452–459, with permission.)
The initial approach includes a focused history and physical examination in combination with noninvasive imaging. Special attention should be directed at documenting the access history including procedures, revisions, and associated complications. Physical examination should include a detailed pulse examination with an Allen’s test to determine the forearm vessel responsible for the dominant arterial supply to the hand. The noninvasive testing in the diagnostic vascular laboratory includes examination of both the arterial and venous circulation. The arterial studies include blood pressure measurements of the brachial, radial, ulnar, and digital arteries along with the corresponding Doppler waveforms of all but the digital vessels (Fig. 2). Additionally, the Allen’s test is repeated, and the diameters of both the radial and brachial arteries are measured at the wrist and antecubital fossa, respectively. Venous imaging includes the interrogation of the cephalic and basilic veins from the wrist to the axilla complete with diameter measurements, similar to the preoperative vein survey obtained prior to infrainguinal arterial revascularization (Fig. 3).
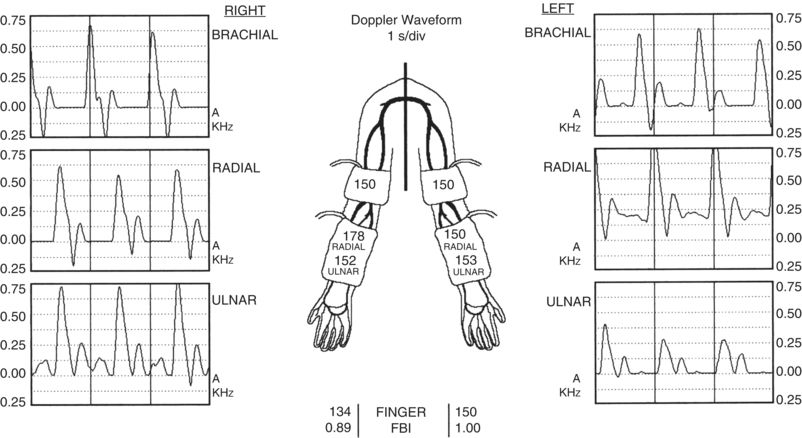
FIGURE 2 Part of the preoperative noninvasive arterial imaging studies is shown. The brachial, radial, and ulnar arterial pressures (mm Hg) are shown on the diagram of the upper extremities while the finger pressures are shown in the center of the figure at the bottom. The corresponding Doppler waveforms (s/div) are shown for the brachial, radial, and ulnar arteries. The FBI denotes the finger/brachial index and is the ratio of the finger pressure to the ipsilateral brachial artery pressure. Note the symmetric brachial artery pressures and the corresponding normal appearing triphasic Doppler waveforms.
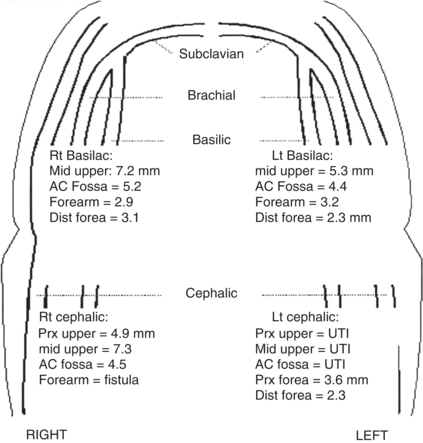
FIGURE 3 Part of the preoperative noninvasive venous imaging studies is shown. The diameters (mm) of both the basilic and cephalic veins are shown on the diagram of the upper extremities. The diameters are reported for the corresponding anatomic segment (prx upper, proximal upper arm; mid upper, mid upper arm; ac fossa, antecubital fossa; dista forea, distal forearm). Note that the basilic vein segments in both upper arms and the cephalic vein in the right upper arm are suitable for autogenous access using the diameter criteria (≥3 mm). The cephalic vein in the left upper arm was unable to be imaged (UTI). Additionally, the patient has a patent access in the right forearm that was not imaged.
An operative plan is then generated based upon the results of the history, physical examination, and noninvasive imaging with a strong emphasis on autogenous access. The objective is to select the combination of the artery and vein that most likely result in a successful arteriovenous fistula (AVF). The usual conventions of using the nondominant > dominant extremity and the forearm > arm are not always pertinent, although I have followed these standard approaches when the choices are equivocal. The criteria for an adequate artery and vein include an adequate diameter, no hemodynamically significant arterial inflow stenoses, no venous outflow stenoses, and a peripheral vein segment of suitable length and diameter (Table 2). My preferences in descending order include the radial-cephalic, radial-basilic, brachial-cephalic, and brachial-basilic autogenous accesses prior to use of prosthetic material (Table 3). Notably, these preferences are consistent with the current KDOQI guidelines.
TABLE 2. Critical Steps to Determine Suitability of Artery and Vein for Autogenous Access

a ≥15 mm Hg pressure gradient between the brachial arteries for proposed arm accesses or between the ipsilateral brachial and radial arteries for proposed forearm accesses.
TABLE 3. Hierarchy for Permanent Hemodialysis Accesses Configurations

Contrast arteriography and venography can be used to confirm the preliminary access choice and are particularly helpful for those with complex problems. Similar to most other applications in vascular surgery, the contrast imaging has evolved from catheter-based procedures to computed tomography (CT)-based procedures. CT venography has the potential ability to image the central venous runoff from all four extremities with a single study, although I have experienced some difficult with the timing of the contrast deliver, particularly in patients with limited peripheral venous access.
Case Continued
The patient undergoes noninvasive imaging as outlined above. She is found to have an axillary vein occlusion on right although the axillary vein on the left appears to be patent. She has evidence of a thrombosed brachial-basilic autogenous access on the right and no suitable peripheral veins (i.e., cephalic or basilic) on either side for an autogenous access based upon the criteria outlined above. Her brachial and radial arterial pressures are normal and symmetric bilaterally with the diameter of the brachial arteries both being greater than 4 mm.
Workup (continued)
The noninvasive imaging suggest that further access attempts are precluded on the right upper extremity based upon the axillary vein occlusion (and the known history of a central vein occlusion based upon previous fistulagram attempts). The left axillary vein appears to be patent and, thus, suitable for a potential access although ultrasound interrogation of the central veins proximal to the axillary vein is limited due to the bony thoracic cavity. The patient has no suitable peripheral veins for an autogenous access; thus, the only potential traditional access option would be a brachial-axillary prosthetic access with the venous anastomosis site more proximally on the axillary vein than the previous one that became infected, necessitating excision. Further imaging with either a CT venogram or catheter-based venogram is necessary to confirm the patency of the central veins on the left.
Case Continued
The patient undergoes a CT venogram that demonstrates that the internal jugular, subclavian, and brachiocephalic veins are occluded on both sides. The superior vena cava appears to be patent but is filled predominantly from collaterals. There is a crushed, thrombosed stent in the right subclavian vein that extends into the brachiocephalic vein. The axillary vein on the left appears to be patent, as suggested by the noninvasive studies. There is a dialysis catheter in the right common femoral vein that extends into the inferior vena cava. The common femoral, external iliac, and common iliac veins are patent on both sides.
Workup (continued)
The CT venogram findings suggest that further attempts at an upper extremity access are relatively contraindicated. However, the study suggests that the lower extremity central veins are patent and that a lower extremity access may be an option. Similar to my generic access algorithm outlined above, noninvasive imaging of the lower extremities is obtained with ankle/brachial indices and duplex ultrasound of the saphenous and femoral system prior to formulating an initial operative plan.
Case Continued
The ankle/brachial indices are not able to be obtained because the tibial vessels are noncompressible. However, the pedal waveforms are triphasic, and the toe/brachial indices are 0.65 bilaterally. The duplex ultrasound of the lower extremities demonstrates that the saphenous and femoral veins are widely patent bilaterally. The femoropopliteal veins are greater than 7 mm, and the saphenous veins are greater than 3 mm. No evidence of venous reflux is seen in either extremity.
Diagnosis and Treatment
Central vein stenoses or occlusions are likely the most common etiology of patients with complex access issues as noted above. The obvious solution is to simply select an alternative extremity with a patent central vein runoff without evidence of stenosis. However, this is not always an option given the widespread use of tunneled dialysis catheters and the prevalence of central vein lesions. I have strongly favored upper extremity accesses over lower extremity ones given the higher incidence of infectious complications associated with the latter, even when this mandates addressing the central vein lesion. The options for placing an access ipsilateral to a known central vein stenosis or occlusion include bypassing or correcting the lesion at the time of access creation or delaying until when/if the venous hypertension and arm edema become problematic. Notably, it is worth emphasizing that a central vein stenosis or occlusion is only a relatively contraindication to an ipsilateral access. Indeed, there are several patients in my current practice that have known central vein occlusions and a functional access with minimal arm edema, presumably secondary to their significant collateral network. Unfortunately, it is difficult to predict preoperatively which patients will develop sufficient collaterals to overcome any significant stenosis, and, thus, I have favored addressing the lesions preemptively. Importantly, superior vena cava syndrome is an absolute contraindication to an upper extremity access.
There are both open and endovascular options for treating the central vein stenosis or occlusions. Similar to most applications, the endovascular options are less invasive but tend to be less durable. The technical success rates for the endovascular approaches are quite good, provided that the lesion can be crossed with a wire. However, the primary patency rates are only about 50% and 25% at 6 and 12 months, respectively. It is unclear whether stents improve the patency rates associated with angioplasty alone, but they are susceptible to being crushed when placed in the thoracic outlet. The patency rates may be improved with covered stents (compared to bare-metal stents), but they have the potential to compromise the venous return through collaterals or the main channels (e.g., internal jugular vein, contralateral brachiocephalic vein) if maldeployed. Multiple open surgical options have been described including axilloaxillary bypass, axillojugular bypass, jugular vein turndown, axilloatrial bypass, and axillofemoral bypass with the potential options largely dictated by the patent vessels. However, the long-term patency rates of these reconstructions are poorly described given their relative infrequency. I have had a modest amount of success with the axillojugular bypass (i.e., ipsilateral) although it is rarely an option since the jugular vein is the preferred site for tunneled dialysis catheters and usually occluded by the time patients are referred to my clinic for their complex access issues.
The HeRO (Hemosphere, Inc., Minneapolis, MN) is a hybrid graft/catheter composed of a 6-mm PTFE graft and a 5-mm (i.d.) nitinol-reinforced silicone catheter that may be useful in the setting of central vein stenoses or occlusions. The system is essentially a prosthetic graft that is connected to central venous catheter that functions similar to a prosthetic access from a patient standpoint. The catheter component is mated to the graft component with a coupling mechanism and serves to provide the outflow from the graft into the atrium or superior vena cava, overcoming the central vein stenosis or occlusions. Insertion of the device requires passing the catheter component (19 French, 6.3 mm o.d.) through the stenotic or occluded central veins. The initial report documenting the outcome of the device reported primary and secondary patency rates of 39% and 72%, respectively, at a mean follow-up of 8.6 months with a bacteremia rate of 0.70/1000 days. It has been my anecdotal impression that the infectious complication rates are significantly higher and the patency rates are lower, but I would readily concede that I have only used the device in a very complex group of patients with extremely limited options, and, thus, my poor results may reflect patient selection rather than limitations of the device.
The permanent hemodialysis access options in the lower extremity are somewhat limited and associated with a higher incidence of ischemic and infectious complications. The most common configuration is a prosthetic thigh loop based off the femoral vessels. I prefer to use the common femoral artery and vein, but others have reported various combinations of the vessels, including the superficial femoral artery and saphenous vein. A recent large series reported primary and secondary patency rates at 12 months of 54% and 75%, respectively, with an infectious complication rate of 27%. Notably, the ischemic complication rate in this series was only 2%, but the low rate likely reflects the authors’ selection criteria. Notably, other authors have reported that infectious complication rates for the lower extremity prosthetic accesses are so high that a tunneled dialysis catheter is a better, safer long-term option. The autogenous options include using either the saphenous vein or the femoropopliteal vein. Although there are several early reports, the saphenous vein is rarely an option. The wall of the saphenous vein is relatively thick (compared to the cephalic and basilic vein), and, thus, it does not dilate much in response to the hemodynamic forces associated with the arteriovenous fistula. Furthermore, it is rarely large enough in terms of diameter (i.e., 5 or 6 mm) to sustain effective dialysis. The femoropopliteal vein can be mobilized and used for an autogenous access, but the wound and ischemic complications are prohibitive and likely exceed those for the prosthetic alternatives.
A number of exotic access configurations have been reported (e.g., axillary artery–renal vein prosthetic access), and, theoretically, it is possible to construct a prosthetic arteriovenous access given a suitable arterial inflow and venous outflow. The long-term durability of these exotic options remains undefined but is likely somewhat limited.
Case Continued
The patient undergoes a left common femoral artery–common femoral vein prosthetic access with 6-mm PTFE.
Surgical Approach
The surgical approach for the femoral-based prosthetic access is fairly straightforward and familiar to most vascular surgeons. I prefer a vertical incision over the femoral artery and try to keep it caudal to the inguinal crease. Some surgeons prefer a transverse or oblique incision, believing that it is associated with a lower incidence of wound complications, although it is my impression that the exposure with the transverse is inferior, particularly for reoperations. The common femoral artery and vein are dissected free sufficiently to apply a side-biting or partially occluding clamp (e.g., Satinsky clamp). In the presence of significant calcification in the common femoral artery, it may not be possible to use a side-biting clamp, necessitating dissecting and occluding the common, profunda, and superficial femoral arteries individually. As noted above, I prefer to site the venous anastomosis on the common femoral vein based upon the logic that it facilitates the largest venous outflow for the anastomosis and, thus, is less likely to develop significant intimal hyperplasia that threatens the graft. The proposed course of the loop graft is marked on the skin, and a separate stab wound is made at the 6 o’clock position to facilitate passing the graft. Alternatively, two separate incisions (i.e., 4 and 8 o’clock) may be made to facilitate tunneling the graft with the choice based upon the patient’s body habitus. The graft is tunneled using a curvilinear tunneling device. The tunnel should be created immediately deep to the dermis throughout the extent that will be used for cannulation but sufficiently deep to the soft tissue in the groin to facilitate wound closure. I typically use a 6-mm PTFE graft but will occasionally use an 8-mm graft in patients with larger femoral vessels and no evidence of arterial occlusive disease, based upon the impression that the patency rates are better. The arterial and venous anastomoses are performed in a standard end-side fashion.
Case Continued
The patient returns to clinic on her 7th postoperative day with some mild redness and swelling at the site of her surgical incision. Ultrasound of her groin demonstrates a small amount of fluid surrounding the graft.
Postoperative Management—Initial
Patients are routinely seen in clinic 2 weeks after their procedure or earlier as dictated by any active access-related issues. Patients with prosthetic access are subsequently seen approximately 30 days after their procedure, and their access is cleared for cannulation provided that there are no new issues. I have generally waited 4 to 6 weeks before cannulating prosthetic accesses although it is likely safe to cannulate them a bit earlier, and this situation occasionally arises in patients who are having difficulties with their tunneled catheters. There are a variety of “early cannulation” grafts commercially available that afford the theoretical appeal of eliminating or reducing the need for tunneled catheters. However, most patients cannot tolerate the discomfort of having their graft cannulated early secondary to the pain and trauma related to the incision and tunneling process. As noted above, infectious complications are common after lower extremity access procedures, albeit usually related to cannulation, and I have taken an aggressive approach in terms of initiating antibiotic treatment. It is common to develop seromas or fluid collections around PTFE grafts. These seromas can lead to graft infections, but they are usually self-limited.
Case Conclusion
The patient’s mild cellulitis resolves in response to the antibiotics. Her access is cleared for cannulation at 4 weeks, and a diagram, with instructions, is sent to her dialysis center.



