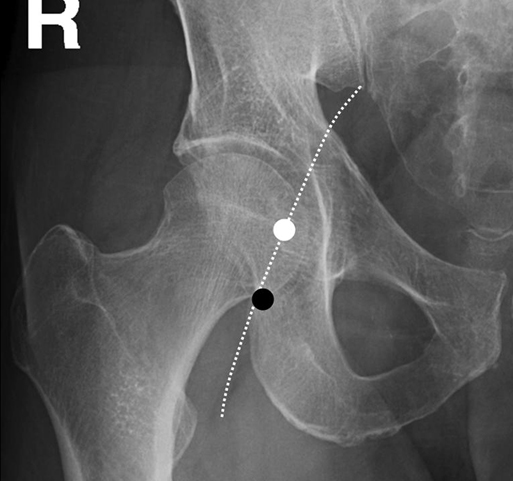
Thabele M. Leslie-Mazwi, Ronil V. Chandra, Daniel C. Oh, Albert J. Yoo and Joshua A. Hirsch The first step in catheter-based angiography of the cerebral vessels is safe arterial access. This is most typically accomplished through a transfemoral route. The common femoral artery is punctured at the femoral head (Figure 1).
Arteriographic Evaluation of Cerebrovascular Disease
Angiographic Procedures
Access to the Arterial System
Arteriographic Evaluation of Cerebrovascular Disease



