Chapter 81
Arterial Tumors
W. Andrew Oldenburg
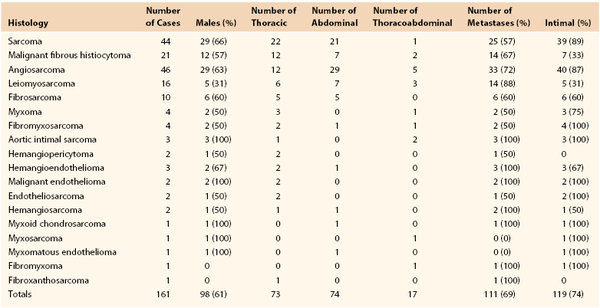
Primary tumors originating in the wall of large arteries and veins are exceedingly rare and frequently are manifested late, making effective treatment problematic. Brodowski described the first case of a primary aortic tumor in 1873.1 When the preceding edition of this book was published in 2010, only 146 primary aortic tumors had been described in the world literature. As of June 2012, an additional 18 cases had been reported (Table 81-1). The most common artery involved is the aorta, but primary arterial tumors have been reported in the iliac,2 subclavian,3 carotid,4,5 renal,6 splenic,7 and popliteal8 arteries. These tumors begin insidiously and frequently mimic other disease processes, resulting in delays in diagnosis and poor outcomes.
This chapter deals predominantly with primary tumors originating in the walls of large arteries. For information on primary tumors involving the large veins, especially the vena cava, see Chapter 65.
Classification of Primary Arterial Tumors
Theoretically, any cellular components of the artery wall can undergo malignant degeneration, although the majority of vascular tumors described in the literature derive from cells originating from the adventitia and media (i.e., sarcomas). Rarely, tumors can originate primarily from the intima. Major arteries can be either primarily or secondarily involved by tumor. More commonly, spread of other malignant neoplasms—gynecologic, head and neck, pulmonary, musculoskeletal, colon, and rectal cancers—affects arteries secondarily. Typically, these tumors extend to the vessel wall and may infiltrate the adventitia; rarely do they extend intraluminally. The major exception to this rule is renal cell carcinoma, which has a proclivity to grow into the renal artery and vein and the inferior vena cava. Most primary tumors of large arteries occur de novo, although several cases have occurred after polyester grafting.9–13 Experimentally, polyester has been shown to induce tumor formation in animal models.14,15 With so few cases being reported in humans, however, a coincidental rather than a causal relationship is suggested.
Morphologic Types
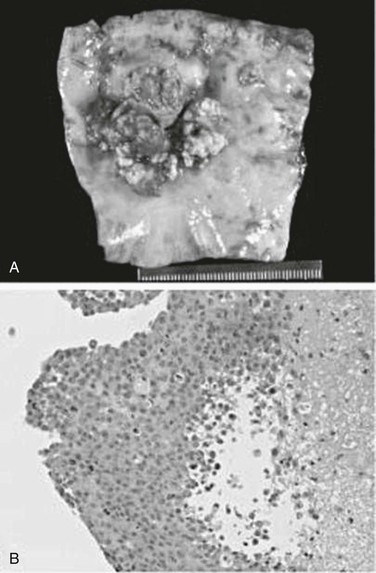
Various classification schemes of aortic tumors have been described, with little change during the past 30 years (Box 81-1). In 1972, Salm described three distinct morphologic types of primary aortic tumors: polypoid (or intraluminal), intimal, and adventitial.16 Polypoid tumors, such as myxomas, have a focal attachment to the intimal surface, with most of the growth extending into the lumen of the vessel (Fig. 81-1). Because of this projection into the lumen of the artery, tumor embolization is common. Intimal tumors grow along the endothelial surface of the aorta, with less pronounced projections into the lumen, leading to large-branch vessel occlusion. As the name implies, adventitial aortic tumors originate in the media or adventitia and grow outward, invading surrounding structures. Wright and colleagues simplified this classification in 1985 by combining the polypoid and intimal groups into intimal tumors and labeling adventitial tumors as mural tumors.17 More than 70% of primary aortic tumors are of the intimal type, with roughly equal distribution throughout the aorta (see Table 81-1).
Histopathologic Types
In the past, histopathologic diagnosis of primary arterial tumors was based on gross appearance as well as light and electron microscopic findings. Frequently, these tumors are so advanced and so poorly differentiated that histopathologic diagnosis is difficult. This has led to a confusing array of pathologic diagnoses, as shown in Table 81-1. Today, with the advent of immunohistochemistry, the pathologic classification of arterial tumors is more precise. Vascular endothelial antigenic stains for von Willebrand factor, CD31 and CD34, and Ulex europaeus agglutinin-1, in association with an atypical spindle cell morphology, are suggestive of intimally based neoplasms such as angiosarcoma and intimal sarcoma. Positive antigenic staining for desmin, smooth muscle actin, and vimentin suggests a tumor originating from the adventitia or media, such as leiomyosarcoma. Metastatic lesions can be ruled out by the absence of immunohistochemical staining for S-100 protein and HMB-45 (melanoma); chromogranin, synaptophysin, and neuron-specific enolase (neuroendocrine tumors); carcinoembryonic antigen and epithelial membrane antigen (epithelial tumors); and CD20, CD30, CD43, and CD99 (hematolymphoid tumors). With better classification of these tumors, cell type–specific adjuvant treatments may have more success.
Diagnosis
Because of the rarity of these tumors as well as the central location of the arteries involved, the diagnosis is difficult and frequently delayed. Less than 5% of all primary aortic tumors have been diagnosed preoperatively. Metastatic disease is identified in more than 60% of patients at the time of initial diagnosis (see Table 81-1). Hence, the majority of patients have nonspecific symptoms such as malaise, fatigue, weight loss, and nausea. Patients with intimally based tumors commonly present with symptoms from tumor embolization, such as blue toe syndrome, an acutely ischemic arm or leg, and acute renal or mesenteric ischemia. The diagnosis is commonly made after pathologic review of an embolectomy specimen, underscoring the need for routine histologic analysis of all embolectomy material. Despite this recommendation, fewer than 70 cases of nonmyxomatous arterial tumor emboli have been described in the literature.18 At the time of vascular reconstruction, primary arterial tumors have been mistakenly diagnosed as an atherosclerotic process,19 aortic aneurysm,20 aortic pseudoaneurysm,21 or aortic dissection.22,23
Conventional arterial imaging, such as ultrasonography, computed tomography, and arteriography, can detect intraluminal filling defects but cannot characterize the lesion or differentiate a degenerative or thrombotic process from a malignant neoplasm. Being invasive, arteriography also carries the risk of precipitating tumor embolization, especially in patients with intimally based tumors. Primary intimal sarcoma of the aorta has been diagnosed with transesophageal echocardiography on the basis of the finding of an inhomogeneous and echo-dense mass with an outer membrane that is atypical for thrombus.24 Magnetic resonance imaging (MRI) may be the most sensitive modality for detecting a primary aortic tumor. MRI has the advantage of providing multiplanar structure imaging, which can differentiate tumor from atheromatous material by enhancement of the tumor on T1-weighted and T2-weighted images without the risk of embolization (Fig. 81-2).25 In addition to tumor enhancement, other MRI features suggestive of tumor include vessel distention by a soft tissue mass or extravascular spread and lack of the abrupt luminal narrowing one would typically see with embolization.26
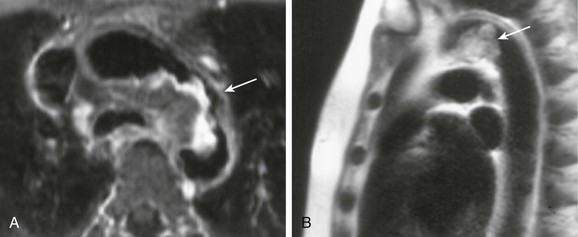
Figure 81-2 MRI of an intimal sarcoma of the thoracic aorta. A, Axial gadolinium-enhanced, fat-saturated, T1-weighted, spin-echo image demonstrating peripheral enhancement (arrow). B, Half-Fourier acquisition, single-shot, turbo spin-echo image showing high T2 signal within the mass (arrow). (Modified from Mohsen NA, et al: Intimal sarcoma of the aorta. AJR Am J Roentgenol 175:1289-1290, 2000.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree