Arterial Disease
I. Magnitude of the problem.
Peripheral arterial disease (PAD) is a leading cause of death worldwide. As an age-related process, PAD will become much more prevalent in decades to come. Specifically, the first of the 82 million Americans in the “baby boom” demographic turned 60 years old in 2006, and the number of people over the age of 65 is anticipated to increase 100% by 2032. Perhaps as important as mortality is the disability resulting from PAD. For example, approximately 750,000 Americans have strokes annually, and many are left with a permanent neurologic deficit, which is devastating to both the patient and the family. Patients with arterial disease may also be limited by chest pain (angina pectoris), exertional leg pain (claudication), extremity ulceration (tissue loss), and even amputation (Chapter 14).
Advances in the diagnosis and successful treatment of PAD have occurred rapidly, and now include broader use of noninvasive duplex ultrasonography (US) and minimally invasive
endovascular techniques (Chapters 10, 11, and 12). Significant progress has also been made in the identification of clinical risk factors and development of new classes of medications that allow prevention or reduction of arterial disease in many patients (Chapter 7). Despite these advances, many patients are unwilling to modify their lifestyles or comply with medication use. Thus, arterial disease will remain a leading health problem during the career of most physicians practicing today.
endovascular techniques (Chapters 10, 11, and 12). Significant progress has also been made in the identification of clinical risk factors and development of new classes of medications that allow prevention or reduction of arterial disease in many patients (Chapter 7). Despite these advances, many patients are unwilling to modify their lifestyles or comply with medication use. Thus, arterial disease will remain a leading health problem during the career of most physicians practicing today.
II. Anatomy.
The peripheral arterial system refers to noncardiac arteries, including the thoracic and abdominal aorta and branches thereof, as well as arteries of the extremities. The arterial network is a complex organ system that must withstand the stress of pulsatile flow for the life of an individual. The wall of the artery consists of three layers, or tunics, referred to as the tunica intima, tunica media, and tunica adventitia (Fig. 1.1). Each of these layers plays a unique part in arterial function to allow delivery of oxygenated blood throughout the body. Although the makeup of each layer varies slightly, depending on the location of the artery in the body, all must function and remain intact in the setting of health.
A. The intima.
The inner lining of the artery is a single layer of endothelial cells called the intima. Endothelial cells of the intima perform unique functions via receptors on their cell surfaces by secretion of proteins, such as endothelin, and other substances, such as nitric oxide, which regulate vessel tone and affect platelet aggregation and formation of thrombus. Like other cells in the body, the cells of the intima require oxygen for survival and function, and receive this from the flowing blood (i.e., luminal blood supply). Underlying the intima is a thin matrix of elastic fibers called the internal elastic lamina.
B. The media.
The middle layer of the artery is formed by a circumferential layer of smooth muscle cells and variable amounts of elastin and collagen. The amount of elastic tissue within the media decreases proportionally to the smooth muscle content as arteries become more peripheral (e.g. further from the heart). Central arteries such as the thoracic aorta with greater elastic content are termed elastic arteries while muscular arteries, such as the femoral or carotid arteries, have greater smooth muscle content in the media. The media primarily responds to signals from endothelial cells of the nearby intima. Under normal conditions, the media provides structure to the vessel and is responsible for variations in vessel tone. In the setting of injury or disease, the media is the main location for cellular response including proliferation of smooth muscle cells and migration of other cell types into the media (e.g., macrophages and fibroblasts). The media has a dual source of nourishment receiving oxygen via diffusion from the circulating blood (luminal oxygen supply) and by small vessels that penetrate the outer wall, which are termed vasa vasorum (abluminal oxygen supply). A second external elastic membrane encloses the outer border of the media and separates it from the adventitia.
C. The adventitia.
The outermost layer of an artery is called the adventitia and is composed mainly of the long fibrous structural protein called collagen as well as autonomic nerves that supply the smooth muscle cells of the media. Additionally, the vasa vasorum courses along and through the adventitia. Although the adventitia may appear thin and without substance, it is a key element in the total strength of the arterial wall. In muscular arteries the adventitia may be as thick as the media itself. In such arteries, surgical closure of the arterial wall or anastomosis of a synthetic graft to the vessel should incorporate the adventitial layer; failure to do so may result in anastomotic breakdown.
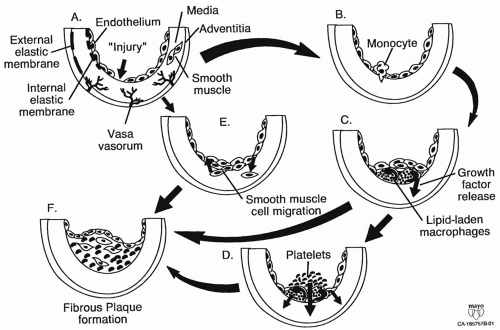 Figure 1.1. Pathogenesis of atherosclerosis. Endothelial “injury” or dysfunction can be initiated by a variety of forces: hyperlipidemia; free radicals caused by cigarette smoking, hypertension, and diabetes mellitus; genetic alterations; elevated plasma homocysteine. Monocytes (A) attach to injured endothelium (B), secrete growth factors (C), and finally migrate into the subendothelial layer. Lipid-laden macrophages become part of the fatty streak. Endothelial disruption attracts platelets (D) that secrete platelet-derived growth factor (PDGF). Smooth muscle cells in the proliferative atheromatous lesion may also secrete growth factors such as PDGF. Increased endothelial turnover results in enhanced growth factor production. Smooth muscle cells are stimulated to migrate into the intimal layer (E). Smooth muscle and “injured” endothelial cells turn up their growth factor production. Fibrous plaques (F) evolve from fatty streaks. Atheromas develop from fatty streaks to fibrous plaques that can degenerate eventually into complicated plaques with surface ulceration, hemorrhage, and embolization. This fibrous plaque rupture and ulceration appear to be related to macrophages releasing proteolytic enzymes. (Adapted from Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115-126.) |
III. Etiology.
The etiology of nearly all acquired arterial disease is atherosclerosis. The term atherosclerosis comes from the Greek terms athero, meaning gruel or paste, and sclerosis, meaning hardening. Arteriosclerosis refers to any hardening of the artery or loss of its elasticity, and is often used interchangeably with atherosclerosis, although technically atherosclerosis is a form of arteriosclerosis.
The etiology of atherosclerosis is a complicated immunemediated process that begins at the interface of endothelial cells
and the circulating blood. The etiology has not been linked to a single causative factor, but rather to a combination of mechanical (e.g., shear stress and hypertension), circulating (e.g., lipids, glucose, or insulin), and environmental (e.g., tobacco use) factors. Together these factors reach a threshold in certain genetically predisposed persons and initiate the disease process of atherosclerosis. The etiology of atherosclerosis may be thought of generally in two stages.
and the circulating blood. The etiology has not been linked to a single causative factor, but rather to a combination of mechanical (e.g., shear stress and hypertension), circulating (e.g., lipids, glucose, or insulin), and environmental (e.g., tobacco use) factors. Together these factors reach a threshold in certain genetically predisposed persons and initiate the disease process of atherosclerosis. The etiology of atherosclerosis may be thought of generally in two stages.
A. Response to injury.
Under normal circumstances the endothelial lining of the blood vessel provides a nonsticky surface through which circulating blood may flow. However, this fragile layer of cells may be damaged by mechanical forces such as hypertension or circulating factors such as metabolites from cigarette smoke or oxidized lipids. Damage to the endothelial cells causes the lining of the vessel to become sticky, and white blood cells (monocytes and T cells) and platelets (thrombocytes) begin to adhere in an attempt to patch the injury (Fig. 1.1 A and B).
Experimental and clinical observations also indicate that early endothelial injury is more prone in areas of blood-flow separation and low shear stress




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


