Chapter 129
Arterial Aneurysms
General Considerations
David H. Deaton
Based on a chapter in the seventh edition by Peter F. Lawrence and David Rigberg
The term aneurysm describes dilatation of any blood vessel. Arterial aneurysms occur throughout the body but are most prevalent in the infrarenal aorta. These aneurysms represent the primary cause of the significant death and disability attributed to arterial aneurysm disease. Aortic aneurysm disease was responsible for approximately 13,000 deaths in 1997.1 Generally speaking, the incidence of aneurysms increases with increasing age, but dangerous aneurysm disease can occur at any age as the result of multiple degenerative processes, including inflammatory, infectious, genetic, and traumatic processes. Whereas aortic aneurysms can cause a variety of symptomatic clinical conditions (e.g., embolism, obstruction of adjacent hollow viscera), the primary danger is from rupture and uncontrolled hemorrhage leading to death. The risk of rupture is related to both the absolute size of the aneurysm and its size relative to normal diameters on the basis of location, body size, and gender. A variety of other factors, including etiology, growth rate, and aneurysm morphology (i.e., fusiform vs saccular), can also be critical in assessing aneurysm risk in certain circumstances.
Historical Perspective
The presence of arterial calcification and atherosclerotic change has been documented in Egyptian mummies from 3500 years ago.2 The Ebers Papyrus (c. 2000 BC) clearly identifies arterial aneurysm, recommending, “Treat it with a knife and burn it with a fire so that it bleeds not too much.”3 Antyllus repaired aneurysms of the extremities with proximal and distal ligation followed by packing of the aneurysm sac.4 Few advances occurred during the next 1000 years. John Hunter ushered in the era of modern surgery based on a scientific understanding of anatomy and physiology, and his contributions to vascular surgery were many. He studied the collateral circulation resulting from the occlusion of main arteries and in 1785 successfully ligated the superficial femoral artery in the treatment of a popliteal aneurysm. The patient did well and maintained function of his lower extremity.5
Rudolph Matas (1860-1957) made numerous contributions to vascular reconstruction. He was the first to perform endoaneurysmorrhaphy in the treatment of a brachial artery aneurysm in 1888. This procedure augmented the proximal and distal ligation of Hunter with evacuation of the aneurysm sac and ligation of tributaries from within, preserving important collaterals, and remains an important principle in modern surgery.6,7 In 1923, Matas performed the first successful aortic ligation; the patient survived 18 months before succumbing to tuberculosis.8 Little progress was made during the next 2 decades, and Bigger’s summary of surgical therapy for aortic aneurysms at the 1940 annual meeting of the American Surgical Association probably represented the consensus of the era: “Judging from the literature, only a small number of surgeons have felt that direct surgical attack upon aneurysms of the abdominal aorta was justifiable, and it must be admitted that the results obtained by surgical intervention have been discouraging.”9
The modern era of aneurysm therapy was ushered in by Charles Dubost in 1951 with the first successful graft replacement of the aorta with an aortic homograft. The introduction of vinyon N cloth in the construction of aortic prostheses by Voorhees, Jaretski, and Blakemore10 addressed the poor availability of aortic homograft and initiated the era of prosthetic vascular reconstruction. The eventual introduction of polyester, championed by DeBakey, and the introduction of new knitting machine technologies to make seamless grafts of multiple sizes and shapes allowed the development and refinement of surgical techniques in many centers rather than in an isolated few.11 Further refinements in surgical, anesthetic, and critical care in the setting of increasing surgical volume defined the ensuing decades until the 1990s, when many surgical series documented mortality for open aneurysm repair below 5% and some at 1% or lower.
The era of endovascular, or transcatheter, repair of aortic and other peripheral aneurysms was made a clinical reality in 1991 by Juan Parodi when he used available balloon-expandable stents in combination with standard polyester grafts to create a device that could be delivered from the femoral artery within the vasculature to the abdominal aorta, where it was deployed and effectively excluded an infrarenal aortic aneurysm. The first devices approved for use in the United States, the Ancure (Guidant, Menlo Park, Calif) and AneuRx (Medtronic, Santa Rosa, Calif) endografts, were approved by the U.S. Food and Drug Administration in June 1999. Today there are numerous endografts available on the market, and current estimates suggest that more than 70% of all aortic aneurysm therapy is performed with endograft technology. Most currently available endograft technology is suitable only for excluding segments of the aorta without branches that require preservation. Off-label combinations of various endovascular devices and more formal clinical trials of protocol-driven endograft designs for treating the mesenteric and arch segments of the aorta are numerous and represent the most active area of current endograft innovation.
Aneurysms of the upper and lower extremity arteries were the first to be treated, and the development of those techniques dates into antiquity as described earlier. The modern approaches to peripheral aneurysm and nonaortic cavitary aneurysm (e.g., splenic, renal) repair typically use the techniques of bypass after proximal and distal ligation of the aneurysm, and their development has generally paralleled the techniques used for arterial bypass. As with aortic aneurysms, a variety of endografting techniques for peripheral aneurysms have been introduced during the last 20 years, with both self-expanding and balloon-expandable stent-grafts used alone or in combination. Embolization techniques have also been selectively used for the treatment of saccular visceral artery aneurysms.
Aneurysm Classification
Size Definitions
The size required to describe an artery as aneurysmal has been defined as a permanent localized (i.e., focal) dilatation of an artery having at least a 50% increase in diameter compared with the expected normal diameter of the artery in question by the Ad Hoc Committee on Reporting Standards of the Society for Vascular Surgery in 1991.12
Aortic
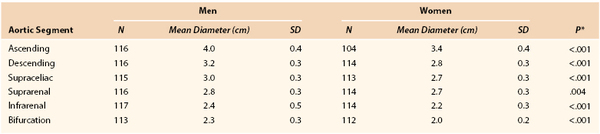
Magnetic resonance imaging of 70-year-old men and women in Sweden determined aneurysmal size and ratio to normal on the basis of a 2 SD difference from normal in the ascending and descending thoracic aorta, supraceliac aorta, and infrarenal aorta (Tables 129-1 and 129-2).
Other definitions for infrarenal aortic aneurysms have used a 3.0-cm threshold for definition across all adults or a 50% increase relative to an adjacent normal-appearing segment.
Peripheral
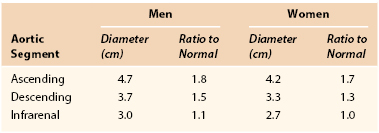
Iliac, popliteal, and femoral aneurysms follow aortic aneurysms in the hierarchy of frequency and are often coexistent with aortic aneurysm, particularly iliac. Aneurysms throughout the rest of the peripheral vascular system, although usually degenerative in nature, may also be related to other pathologic processes, such as arteritis, infection, and connective tissue disorders. The “normal” diameters of a variety of peripheral arteries, as determined from imaging studies, are listed in Table 129-3. Irrespective of the ratio of an aneurysm to its normal arterial diameter, the physical forces of wall tension are dictated by the absolute radius rather than by any ratio to a normal size.
Table 129-3
Normal Diameters of Peripheral Arteries
| Normal Diameter (cm) | |
| Celiac | 0.5 |
| Superior mesenteric | 0.6 |
| Common femoral | 0.8 |
| Popliteal | 0.9 |
| Tibial | 0.3 |
Modified from Johnston KW, et al; Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery: Suggested standards for reporting on arterial aneurysms. J Vasc Surg 13:452-458, 1991.
True versus False Aneurysms
The distinction of true or false aneurysm (usually termed pseudoaneurysm) is dependent on whether the lesion represents a dilatation of the vascular wall (i.e., true aneurysm) or is a locally contained hematoma resulting from disruption of the vessel (i.e., pseudoaneurysm), in which the “wall” of the pseudoaneurysm is actually connective tissue formed in reaction to the contained hematoma. This distinction usually does not affect decisions about therapy as progressive enlargement and its consequences are the indications for intervention in both. Many peripheral pseudoaneurysms are the result of trauma, often iatrogenic during peripheral arterial access required for imaging or therapy (Fig. 129-1). These pseudoaneurysms often have a “neck” or narrow conduit between the affected artery and the main pseudoaneurysm cavity. When this morphology exists, therapy directed at inducing aneurysm thrombosis is often effective in treating the aneurysm.
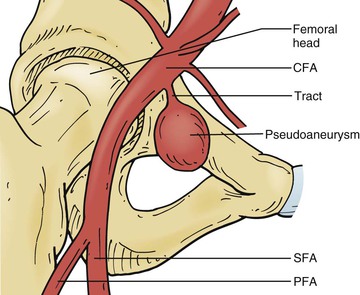
Figure 129-1 Iatrogenic femoral false aneurysm or pseudoaneurysm after percutaneous femoral arterial puncture. CFA, Common femoral artery; PFA, profunda femoris artery; SFA, superficial femoral artery. (From Kronzon I: Diagnosis and treatment of iatrogenic femoral artery pseudoaneurysm: a review. J Am Soc Echocardiogr 10:236-245, 1997.)
Another frequent cause of pseudoaneurysms is the loss of anastomotic integrity at a prior surgical anastomosis. These perianastomotic pseudoaneurysms represent dehiscence of the original suture line from the graft to the vessel (Fig. 129-2). Endovascular techniques have greatly simplified the repair of these lesions in some cases.
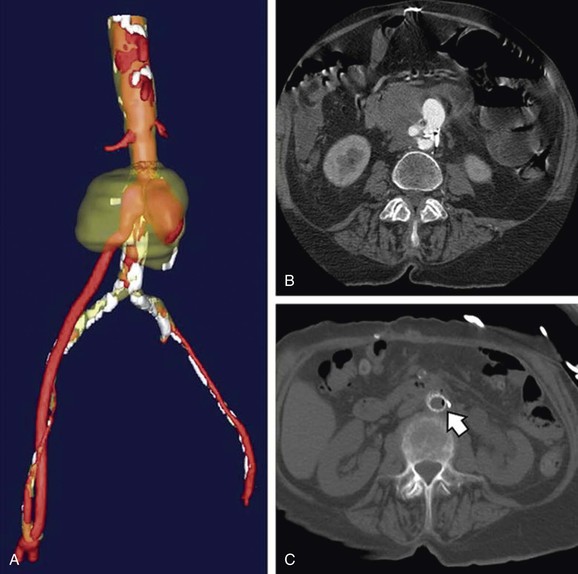
Figure 129-2 Three-dimensional reconstruction (A) and axial computed tomography imaging (B, C) of a proximal infected anastomotic pseudoaneurysm in an aortofemoral bypass graft. Arrow shows gas formed by infecting bacteria. (From Lew WK, et al: Endovascular management of mycotic aortic aneurysms and associated aortoaerodigestive fistulas. Ann Vasc Surg 23:81-89, 2009.)
Location and Extent
Aneurysms are classified by their location (e.g., aortic, splenic) and their extent. Ectasia refers to an intermediate stage of enlargement when an artery is less than 50% enlarged, whereas arteriomegaly refers to diffuse, continuous enlargement of multiple arterial segments dilated to greater than 50% of normal. Arteriomegaly and ectasia are descriptive terms rather than representative of a specific diagnosis and likely constitute part of a not yet clearly defined genetic syndrome of arterial degeneration. They are associated with an increased incidence of clinically significant aneurysm formation and increased risk for aneurysm disease in first-degree relatives.13 The term aneurysmosis is used to describe multiple aneurysms in several anatomic locations or the combination of aneurysmal degeneration in the setting of arteriomegaly.14
Morphology
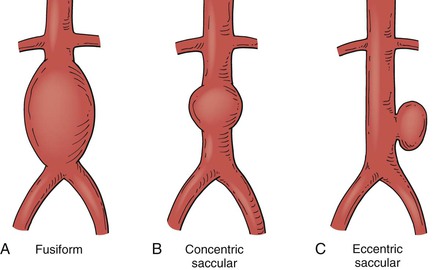
The shape of aneurysms is typically described as fusiform or saccular. Fusiform aneurysms represent a generalized increase in the entire diameter of the affected vessel; saccular aneurysms are more localized. Some saccular aneurysms are eccentric defects arising from a focal location in the arterial wall, often as a result of trauma or infection (Fig. 129-3).
Most peripheral arterial aneurysms are associated with atherosclerotic degeneration, which usually results in fusiform or concentric saccular morphology. Eccentric saccular morphology is typical of intracranial or cerebral aneurysms but is less common in noncerebral locations. However, most pseudoaneurysms have an eccentric saccular shape. Whereas fusiform and concentric saccular aneurysms result from a generalized weakening of the entire circumference of the affected vessel, eccentric saccular aneurysms result from a focal weakness that may be due to an intrinsic abnormality or an extrinsic process causing focal arterial damage. Intrinsic causes associated with eccentric saccular aneurysms generally involve a focal “tear” or partial rupture of the arterial wall at that location. Penetrating atherosclerotic ulcers and intramural hematomas are examples of focal tears in the aortic wall (that fail to propagate down the media, as in the case of aortic dissection) and have a morphology consistent with eccentric saccular aneurysms. These entities have become more recognized in the past decade with respect to their presentation, prognosis, and treatment, particularly as relatively straightforward endovascular techniques are often effective therapies for these lesions.15,16 The risk of rupture is better characterized for fusiform or concentric saccular shapes but is less well understood for eccentric saccular aneurysms. Saccular morphology and associated symptoms are often used as factors in advising intervention at a diameter less than that for fusiform aneurysms. Many saccular aneurysms are easier to treat with endovascular techniques because of their focal nature. Size, growth rate, and amenability to repair are often significant factors in the decision to repair or to observe these unusual lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree