Approaches to the Patient with Prior Bypass Surgery
John S. Douglas Jr.
Overview
The important issues in the patient who has undergone coronary artery bypass grafting (CABG) and who has recurrent angina or ischemia are providing relief with the least morbidity and cost and doing it in a manner that provides durable benefit. It takes considerable judgment to make the best decision in these patients because there are frequently many options, most of which have substantial baggage in the form of complications and short-term benefit.
Historical Perspective
Advent and Maturation of Surgical Revascularization
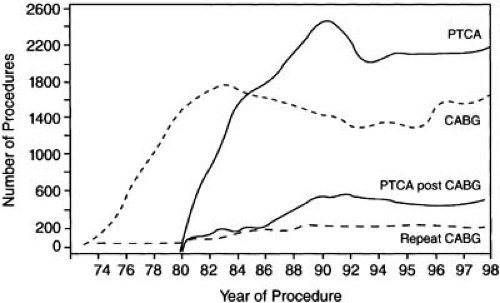
As was later true of percutaneous transluminal coronary angioplasty (PTCA) (1,2), surgical coronary artery revascularization began more than 30 years ago with attempts to revascularize a single coronary artery. The result of these efforts was a dramatic change in cardiologic practice as surgical techniques adequate to palliate multivessel obstructive disease evolved. Within a decade, the growth of CABG surgery was exponential (Fig. 81.1). Hundreds of thousands of patients were operated on annually, creating a large population of several million postbypass patients in the United States alone. Owing to the progressive nature of the atherosclerotic process and the limited durability of venous conduits and in spite of widespread use of arterial grafts and antiplatelet agents, recurrent ischemia after surgical revascularization is a problem all cardiologists face with increasing frequency. Recurrence of angina in the first year alone was reported in 24% of patients in the Coronary Artery Surgery Study, and by the fifth postoperative year, almost one half of the broad spectrum of postoperative patients will have recurrent symptoms (3).
Recurrent angina sufficient to require reoperation occurred in 12% to 15% of patients within 1 decade of a first coronary operation at Emory University in Atlanta, Georgia and at the Cleveland Clinic in Cleveland, Ohio, and by the twelfth to fifteenth year, 30% required reoperation (4,5). Reoperative coronary surgery when compared with initial operation proved to be more costly, two to three times more likely to lead to in-hospital death or myocardial infarction (MI), and less effective in relieving angina. Among 2,030 patients who underwent reoperation at Emory University, in-hospital mortality was 7.0% (4.6% < 60 years, 8.2% aged 60 to 69, 10.0% ≥ 70 years). Five- and 10-year survival was 76% and 55%, respectively (6). Reported outcome data confined to patients who underwent reoperation in the 1990s confirmed the relatively high operative mortality noted earlier (7.4%) and reported a higher rate of Q-wave infarction, a longer length of stay, and higher costs than initial operations (7). The increase in complications and reduced efficacy of reoperative coronary surgery are probably related to the more extensive disease present, the requirement to use second-line venous conduits in many cases, and the technically more difficult and operator-dependent nature of reoperative heart surgery.
Percutaneous Postbypass Intervention
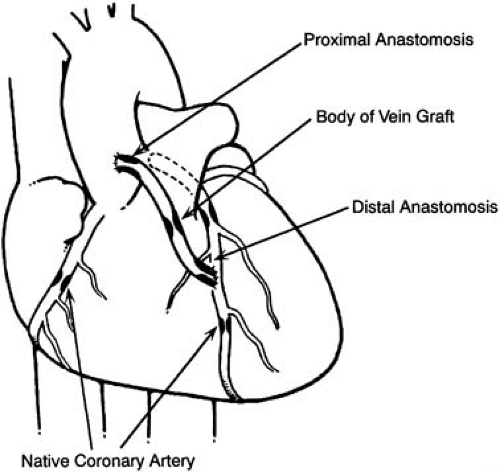
Percutaneous catheter revascularization in patients who had prior CABG surgery (Fig. 81.2) was first described by Gruentzig, who treated 8 patients, 6 successfully, among the first 50 patients he reported. Seven of the 8 postbypass patients had angioplasty of saphenous vein graft (SVG) lesions, and 3 of 5 treated successfully had recurrences, findings that led Gruentzig to make the following insightful comments based on such limited observations: “The different kind of disease may explain the high incidence of recurrence in graft stenosis. Further experience will show whether we should eliminate this lesion from consideration” (8).
Among the first 1,116 patients treated with coronary angioplasty in the National Heart, Lung, and Blood Institute PTCA Registry, 62 patients had had previous bypass surgery. The in-hospital mortality rate in these patients, 8.1%, was significantly
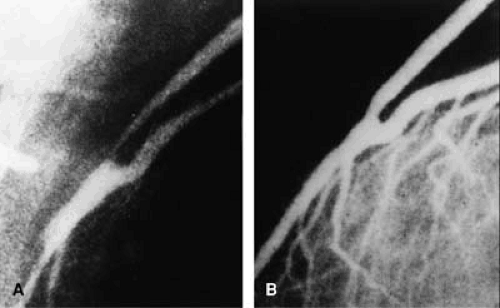
higher than in patients without prior surgery, 0.7%, thus causing concern about the safety of the procedure in this group of patients (9). The early experience at Emory University was reassuring, however; 116 treated patients had no procedural deaths, 3 had emergency operations, and one had a Q-wave MI (Fig. 81.2). One late death occurred during an 8.3-month mean follow-up. Gruentzig’s observation of higher restenosis rates for SVGs was reaffirmed for medial and proximal graft sites, but an acceptable restenosis rate for distal anastomosis lesions of 18% (4 of 22) was first reported (Fig. 81.3), as well as the initial report of attempted internal mammary artery (IMA) graft angioplasty (10). In subsequent experience with more than 34,000 coronary angioplasty procedures at Emory University, a history of prior bypass surgery was not associated with an increased risk of death or Q-wave MI and was negatively correlated with the need for in-hospital CABG surgery (11).
higher than in patients without prior surgery, 0.7%, thus causing concern about the safety of the procedure in this group of patients (9). The early experience at Emory University was reassuring, however; 116 treated patients had no procedural deaths, 3 had emergency operations, and one had a Q-wave MI (Fig. 81.2). One late death occurred during an 8.3-month mean follow-up. Gruentzig’s observation of higher restenosis rates for SVGs was reaffirmed for medial and proximal graft sites, but an acceptable restenosis rate for distal anastomosis lesions of 18% (4 of 22) was first reported (Fig. 81.3), as well as the initial report of attempted internal mammary artery (IMA) graft angioplasty (10). In subsequent experience with more than 34,000 coronary angioplasty procedures at Emory University, a history of prior bypass surgery was not associated with an increased risk of death or Q-wave MI and was negatively correlated with the need for in-hospital CABG surgery (11).
Percutaneous coronary intervention (PCI) became a less invasive revascularization alternative for many symptomatic postbypass patients, including increasing numbers who, because of contraindications (pulmonary and renal failure, old age, malignancy), were not candidates for reoperative coronary artery surgery. Patients with patent arterial grafts that would be jeopardized by reoperation, patients with relatively small amounts of ischemic, symptom-producing myocardium, and patients with no available venous or arterial conduits for grafts underwent percutaneous revascularization at an acceptable risk. At many centers, patients with prior bypass surgery account for up to 25% of the PCI procedures performed.
Anatomic Considerations
Among the anatomic factors influencing revascularization decisions (Table 81.1), the status of the left anterior descending coronary artery (LAD) and its graft are paramount. Placement of an IMA, a conduit immune to atherosclerosis, as a graft to the LAD has been shown to enhance survival and to reduce ischemic events 10 to as long as 20 years later. In a patient with a patent IMA graft to the LAD, reoperative surgery to treat non-LAD ischemia has been reported to offer no survival benefit, may jeopardize the arterial graft, and, in our experience and that of others, more frequently leads to percutaneous intervention. Multivessel involvement, small number of patent grafts, severe vein graft disease (especially if to the LAD), and a moderately impaired left ventricle are factors more likely to lead to reoperative surgery.
Pathophysiology: Basis for Recurrent Ischemia
Incomplete Revascularization
In many patients, complete surgical revascularization was not achieved because of the presence of distal coronary disease,
whereas less common causes include the following: an inadequate amount of venous or arterial conduit, inadequate conduit lumen resulting from small vessel size (IMA especially) or injury, intramyocardial location of target coronary vessel, placement of grafts to wrong coronary artery or to a coronary vein, creation of an arteriovenous fistula, use of an IMA in the presence of significant stenosis of the subclavian or innominate arteries, or coronary steal phenomenon attributed to large arterial graft side branches, a cause that has been questioned. Intentional incomplete surgical revascularization is an increasingly frequent phenomenon in patients selected for “beating heart” operations because of the technical difficulty of bypassing posteriorly located coronary arteries. In some patients, this strategy has resulted in subsequent percutaneous revascularization because of inadequate relief of symptoms. In others, incomplete surgical revascularization has been dealt with up front with adjunctive percutaneous revascularization during the same hospitalization, the so-called hybrid approach. In addition to less complete revascularization with beating heart surgery, there is some evidence that less precise anastomoses may compromise graft patency, especially in the surgical learning curve (see the later discussion of venous graft attrition and arterial graft compromise).
whereas less common causes include the following: an inadequate amount of venous or arterial conduit, inadequate conduit lumen resulting from small vessel size (IMA especially) or injury, intramyocardial location of target coronary vessel, placement of grafts to wrong coronary artery or to a coronary vein, creation of an arteriovenous fistula, use of an IMA in the presence of significant stenosis of the subclavian or innominate arteries, or coronary steal phenomenon attributed to large arterial graft side branches, a cause that has been questioned. Intentional incomplete surgical revascularization is an increasingly frequent phenomenon in patients selected for “beating heart” operations because of the technical difficulty of bypassing posteriorly located coronary arteries. In some patients, this strategy has resulted in subsequent percutaneous revascularization because of inadequate relief of symptoms. In others, incomplete surgical revascularization has been dealt with up front with adjunctive percutaneous revascularization during the same hospitalization, the so-called hybrid approach. In addition to less complete revascularization with beating heart surgery, there is some evidence that less precise anastomoses may compromise graft patency, especially in the surgical learning curve (see the later discussion of venous graft attrition and arterial graft compromise).
TABLE 81.1 Anatomic Factors Influencing Revascularization Decisions in Postbypass Patients | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Loss of Revascularization Benefit
Venous Graft Attrition
In patients with recurrent ischemia and infarction after bypass surgery, stenosis or occlusion of SVGs is the most common cause. Thrombotic occlusion related to surgical technical problems or slow graft flow secondary to a small or compromised distal coronary arterial bed results in closure of 10% to 15% of SVGs within the first month, and even with aspirin therapy, one report noted that 7% of vein grafts were shown to be occluded by 9 days, and up to 17% of patients had a closed graft at that time (12). Subsequently, 15% to 20% of vein grafts occluded by 1 year, 1% to 2% per year from years 1 to 6, and 4% per year from 6 to 10 years after surgery. After 10 years, a few vein grafts were free of significant occlusive disease.
Native Coronary Artery Progression
Worsening of native coronary artery disease after bypass surgery has been reported in about 5% of patients annually (13). Frequency of disease progression at native vessel sites at 5 years was reported as follows: proximal to graft insertion (70%), unbypassed artery (15%), and distal to graft insertion (0%) (14). Loop noted that progression of disease distal to grafts was uncommon, and at 2 years, progression of disease proximal to grafts was more common for SVG than for IMA grafts (67% versus 39%) (15). Progression of native coronary disease proximal to grafts has implications for coronary intervention when graft disease or occlusion occurs and is an important cause of ischemia resulting from poor retrograde perfusion of side branches (e.g., diagonal coronary arteries).
Arterial Graft Compromise
Although numerous publications attest to the excellent long-term patency of arterial grafts, several hundred patients have been reported who required IMA, radial artery, or subclavian artery intervention (16,17,18,19,20,21,22). In most instances, IMA interventions were needed for anastomotic lesions, but proximal and midgraft sites have been treated (see the discussion of arterial graft intervention). The technical difficulty of performing an anastomosis in beating heart surgery probably accounts for more anastomotic problems following this type of surgery. Whether radial artery grafts are superior to SVGs has been questioned.
Principles of Management
Preventive Measures
Patients who have had CABG surgery are at substantial risk for subsequent cardiac events related in large part to progressive arteriosclerosis. Recurrence of angina, angiographic progression of vein graft atheroma, and pathologic changes at autopsy have been correlated with increased serum lipids and smoking, and lipid lowering has been shown to be beneficial (23,24). Given the increased effectiveness of current strategies for lipid lowering and smoking cessation, and reduced cardiac events that accrue, an aggressive approach to risk factor modification is mandatory in all postbypass patients. An aggressive lipid-lowering strategy resulted in a 30% reduction in revascularization and a 24% reduction in a composite clinical end point during 7.5 years of follow-up in the post-CABG Trial. The place of more aggressive antiplatelet therapy in the post-CABG patient is not clear, but recently reported mortality benefits with clopidogrel are provocative and suggest that the use of this or other adjunctive agents may become routine in the future. However, data from randomized controlled trials currently support only aspirin and lipid-lowering agents for routine therapy in all post-CABG patients (25).
Treatment of Ischemia in the Postoperative Patient
Patients with recurrent symptoms or signs of ischemia after bypass surgery constitute an extremely heterogenous group (Fig. 81.2). Treatment strategies must be based on a careful analysis of multiple factors (many angiographically based) including anatomic factors (Table 81.1), the likelihood of a successful percutaneous intervention, risk of complications, probability of long-term symptomatic benefit, and resource consumption compared with other viable options. Patient preferences must be considered because reinterventions are common with percutaneous revascularization in postbypass patients and occur in about 50% of patients at 5 years. In the postbypass patient, the ability to effect ischemia relief percutaneously is influenced by the time that has lapsed since surgery, the type of conduit (SVG versus native vessel versus IMA graft), and the location of the stenotic segment.
Results of Native Coronary Intervention
The procedural outcome of angioplasty for native coronary intervention after CABG was reported for the first 372 such patients treated at Emory University in 1987 (26). Most were men (81%), and 78% had multivessel disease. Angiographic success was achieved in 91%, and in-hospital complications were infrequent: mortality, 0.3%; Q-wave MI, 2%; non–Q-wave MI, 4%; CABG surgery, 6%. At the Mid-America Heart Institute (Kansas City, MO), 1,543 postbypass patients underwent angioplasty of native coronary arteries. Angiographic success was 94%, in-hospital mortality was 0.8%, Q-wave MI was 1.5%, and emergency bypass surgery was 1.0% (27). At Emory University between 1980 and 1995, 2,246 postbypass patients underwent coronary intervention of native arteries with favorable outcome: procedural success, 89%; in-hospital mortality, 1%, Q-wave MI, 1%; non–Q-wave MI 4% (creatine kinase greater than three times normal); and emergency or elective surgery, 2.8%. Although the definitions of outcomes were slightly different in these series, the overall procedural results were favorable, with a trend toward less need for in-hospital bypass surgery for failed percutaneous intervention. These outcomes were largely that of conventional balloon angioplasty. The Mayo Clinic (Rochester, MN) experience with 937 post-CABG patients treated between 1995 and 1998 reflected a dramatic increase in stent use to 76% of patients and a reduction in use of atherectomy and laser strategies. Patients who underwent interventions in native vessels were younger, more likely female, had less severe coronary disease, and had a more favorable long-term outlook than those who underwent venous bypass graft intervention (28). Results of the use of drug-eluting stents in native coronary intervention after CABG have not been reported.
Results of Saphenous Vein Graft Intervention
Numerous reports have described the results of balloon angioplasty in SVG disease, indicating that, in selected patients, success rates of approximately 90% were achieved, with mortality of about 1%, Q-wave MI of less than 2%, and in-hospital CABG in approximately 2% of patients. Many of these patients had relatively favorable anatomy with focal lesions free of obvious thrombus. Non–Q-wave MI, the most frequent complication, occurred in 78 (13%) of 599 patients at Emory University (29,30). The length of time since surgery was an important predictor of restenosis (<6 months, 32%; 6 months to 1 year, 43%; 1 to 5 years, 61%; and 64% for >5 years; p < .02), as was the location of the lesion (proximal anastomosis, 68%; midgraft, 61%; distal anastomosis, 45%; p < .06). The lowest restenosis rate, 22%, was noted for lesions that occurred at the distal anastomosis within 1 year of surgery, and these patients had excellent, event-free survival (Fig. 81.3).