Clinical Approach to the Patient |
The most common respiratory complaints prompting medical evaluation are shortness of breath and cough. Less frequent are hemoptysis and thoracic pain. As in any medical assessment, a detailed history and thorough physical examination are of paramount importance. Use of plain chest radiography for routine screening, once popular in the hope of uncovering silent disease amenable to therapy, is not routinely employed, as it has not been proven to decrease mortality or to be cost effective. Chest radiography is now usually reserved for patients who have clinical manifestations of thoracic disease; serial chest radiographs often provide invaluable clues regarding the underlying problem. More sophisticated imaging techniques, including computed tomography (CT),1,2 along with tests of lung function, help complete the clinical picture.
HISTORY
Although seasoned clinicians may be adept at quickly spotting telltale diagnostic clues, a comprehensive medical history is central to patient evaluation. The history should include a detailed inventory of exposure to air-borne substances that may result in lung injury. One of the most common offenders is cigarette smoke. An attempt should be made to quantify the exposure.
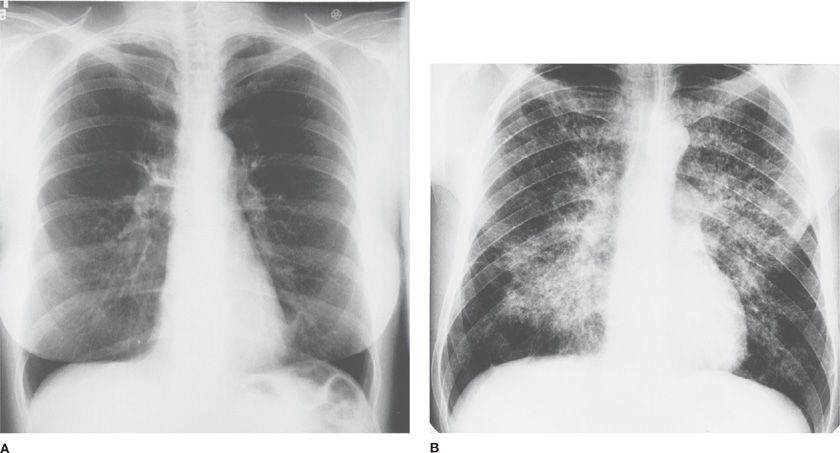
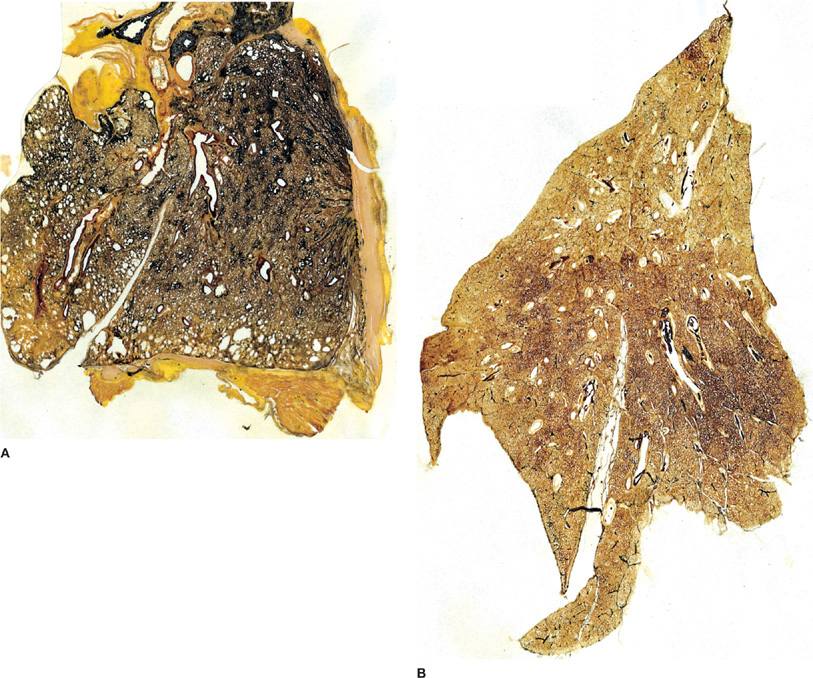
Often, the workplace is the site where toxic air is inhaled. An almost forgotten exposure to a toxic inhalant 20 years ago may explain certain types of pulmonary or pleural diseases. Symptoms that appear to improve during weekends or other periods away from work may be a clue to an occupational exposure that causes a respiratory ailment. A newly installed home humidifier or an air conditioning system that incorporates stagnant pools of water can point the way to resolving a mysterious illness. Brief residence in an area where either cryptococcosis (southwestern United States) or histoplasmosis (southern and midwestern United States) is endemic may help clarify the nature of an illness that mimics tuberculosis. A recent visit to a South or Central American country may bring into focus a more remote possibility (e.g., South American blastomycosis) (Fig. 29-1).
Figure 29-1 Exposure in an endemic area. A. Clear lung fields. B. South American blastomycosis. (Used with permission of Dr. Nelson Porto.)
The history should include a thorough evaluation of prior and current medical problems. Rheumatologic disorders, such as systemic sclerosis (scleroderma), may be associated with interstitial lung disease, aspiration pneumonia due to esophageal involvement, or pulmonary vascular disease. Certain malignancies often metastasize to the lung (e.g., breast or colon carcinoma), or predispose to development of venous thromboembolism (e.g., pancreatic carcinoma). Infection with the human immunodeficiency virus (HIV) should not be overlooked, since pulmonary complications are often the initial presentation of acquired immunodeficiency syndrome (AIDS). Other causes of immunodeficiency, such as hematologic malignancy, or prior administration of chemotherapeutic agents, should heighten suspicion of infection as the cause of respiratory symptoms, as well as potential pulmonary drug toxicity.
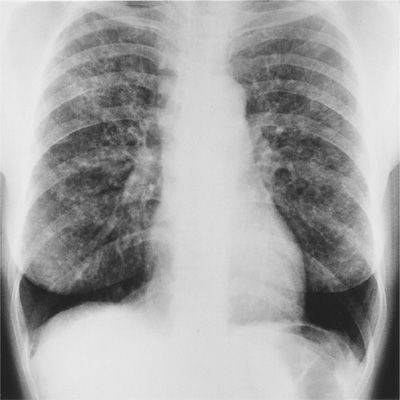
Indeed, many pharmacologic agents, including chemotherapeutic and nonchemotherapeutic agents, have a propensity for inflicting lung damage (see Chapters 65 and 66). Classic examples include bleomycin, nitrofurantoin, and methotrexate (Fig. 29-2). Beta blockers, administered as part of a cardiac regimen, may evoke bronchoconstriction. Even a common medication, such as aspirin, may, on rare occasion, cause a severe pulmonary disorder (e.g., pulmonary edema).
Figure 29-2 Nitrofurantoin hypersensitivity pneumonitis. The ingestion of nitrofurantoin was accompanied by the appearance of patchy interstitial and alveolar changes throughout both lungs.
Finally, the family history is an essential ingredient of the medical inventory. This history can uncover a heritable disease of the lungs (e.g., cystic fibrosis [CF], α1-antitrypsin deficiency, alveolar microlithiasis, and hereditary telangiectasia).
PHYSICAL EXAMINATION
Before the widespread use of chest radiography, physical examination, along with the history, played a pivotal role in the diagnosis of pulmonary disease. The advent of chest radiography and chest CT imaging has de-emphasized the value of the physical examination. Nonetheless, the physical examination remains a valuable diagnostic measure in the appraisal of chest disease.3,4
 GENERAL ASPECTS
GENERAL ASPECTS
Important clues are often available before examination of the chest. For example, neglected pyorrheal teeth raise the prospect of necrotizing aspiration pneumonia. A lacerated tongue suggests that a convulsive episode may have led to aspiration (Fig. 29-3). Pursing of the lips during expiration (“pursed-lip breathing”) may be seen in patients with chronic obstructive pulmonary disease (COPD). Subtle changes in consciousness or coordination may signal that metastasis has occurred to the brain from a primary carcinoma of the lung. In the patient with COPD, a clouded sensorium or a disturbed personality can signify acute elevation in arterial PCO2.
Figure 29-3 Chronic aspiration pneumonia. A. Chronic aspiration pneumonia in a 72-year-old man hospitalized for repair of hernia. Patchy infiltrates bilaterally. No pulmonary symptoms. Initiating cause was achalasia of esophagus. B. Eighteen months later. Persistent cough and breathlessness.
Inspection of the skin often provides clues to diseases of the chest; a more detailed discussion of notable cutaneous manifestations in respiratory disorders is provided later in this chapter. Evidence to support the diagnosis of pulmonary sarcoidosis may be found in the eyes and skin. Petechiae, purpura, necrosis, and/or ulceration of the skin may reflect a systemic vasculitis. The skin lesions of neurofibromatosis type 1 (von Recklinghausen disease) may signify that a solitary pulmonary nodule in the paraspinal region may be a neurofibroma. A minute skin abscess may turn out to be the source of multiple lung abscesses. Distinctive scars over the antecubital veins of a drug addict can help to clarify the etiology of old lesions in the lungs, as well as of a newly discovered lung abscess. Erythema nodosum (EN) is frequently due to sarcoidosis, but may also occur in patients with tuberculosis, histoplasmosis, or coccidioidomycosis. Skin papules in Birt–Hogg–Dube syndrome (see Pulmonary-Cutaneous Syndromes, below) may antedate the pulmonary manifestations of cystic lung lesions and pneumothorax by decades.5
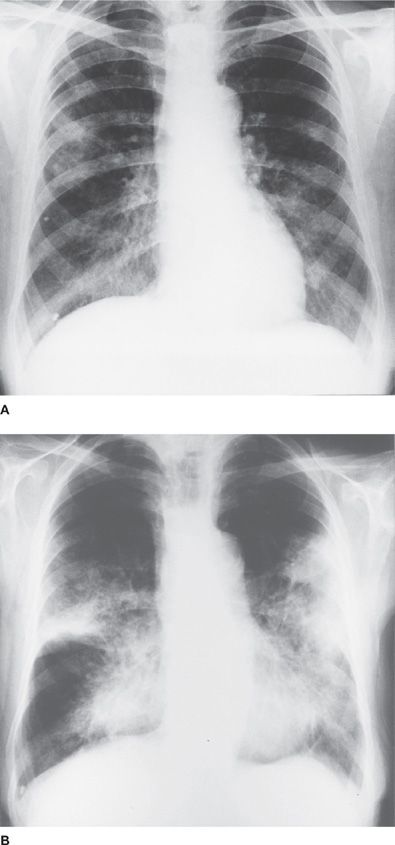
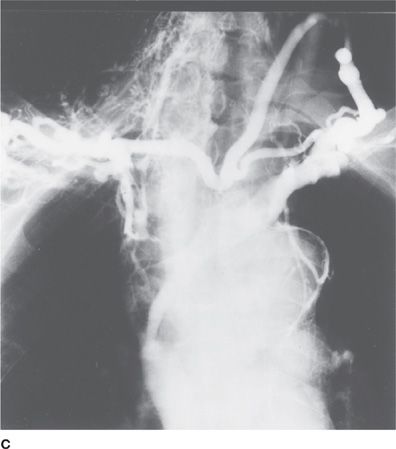
A variety of endocrine syndromes may accompany carcinoma of the lung. An altered mental status may be due to hyponatremia caused by the syndrome of inappropriate antidiuretic hormone (SIADH). Clubbing of the digits may accompany various clinical disorders, including idiopathic pulmonary fibrosis, bronchiectasis, and certain carcinomas of the lung (Table 29-1).6 A puffy face, neck, and eyelids, coupled with dilated veins of the neck, shoulder, thorax, and upper arm (i.e., superior vena cava syndrome) may constitute the first clinical evidence of obstruction of the superior vena cava by a neoplasm of the lung. Although the causes of superior vena cava syndrome are many and diverse, at least 80% are attributable to a primary carcinoma of the lung (Fig. 29-4). In the patient in whom a neoplasm has evoked acute signs and symptoms of increased systemic venous pressure that progresses rapidly (e.g., to laryngeal edema), early diagnosis and prompt treatment of the neoplasm can be lifesaving. The presence of Horner syndrome – unilateral ptosis, miosis, and anhidrosis – in a patient with a carcinoma of the lung suggests a pulmonary sulcus tumor with involvement of the ipsilateral sympathetic pathway within the thorax (Fig. 29-5).
TABLE 29-1 Clinical Disorders Commonly Associated with Clubbing of Digits
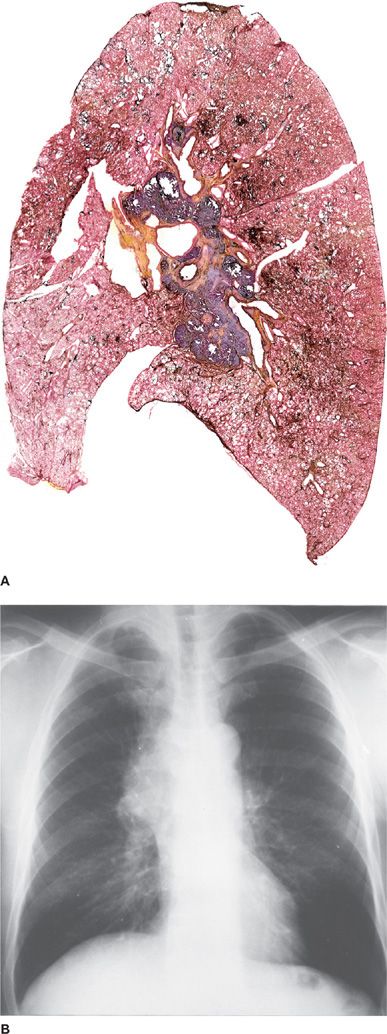
Figure 29-4 Local invasiveness of carcinoma of the lung. A. Sagittal section of the lung illustrating a carcinoma (blue) of the lung in the vicinity of the hilus. B. Chest radiograph showing right hilar mass. C. Angiogram showing obstruction and extensive collateral circulation.
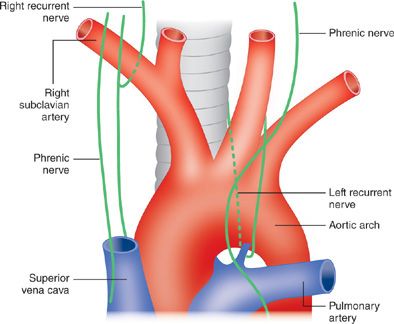
Figure 29-5 Courses of the recurrent laryngeal nerves. Invasion or compression of a nerve by a carcinoma of the lung causes paralysis of the vocal cord.
 INSPECTION OF THE CHEST
INSPECTION OF THE CHEST
Observation of the chest from the foot of the bed can be informative: a visible lag in expansion of one side of the thorax localizes a pleural effusion, pulmonary infection, or paralyzed diaphragm. The respiratory pattern may be informative: patients with severe airflow obstruction often take slow, deep breaths, whereas rapid and shallow breaths are often seen with restrictive processes, such as interstitial lung disease or kyphoscoliosis. Inspection of the chest and abdomen in the supine position may reveal paradoxical inward movement of the abdomen, indicative of respiratory muscle weakness.7
 PALPATION OF THE CHEST
PALPATION OF THE CHEST
Over the years, the role of palpation in examination of the chest has been considerably devalued. Nonetheless, palpation may provide helpful diagnostic clues as well as confirmatory evidence for other physical signs. For example, the position of the trachea determined by palpation in the suprasternal notch may be helpful in detecting a lateral displacement of the upper mediastinum. Displacement of the apical impulse and of cardiac dullness may be useful indices in detecting shift of the lower mediastinum. Tenderness over a rib may reflect a fracture, metastasis, or underlying pleuritis. Enlargement of the right ventricle can be readily detected by palpation in the subxiphoid region. Hoover sign may be useful in disclosing a unilateral lag in motion of one side of the chest due to pleuritis or a pleural effusion. The sign is elicited by comparing the displacement from the midline during a patient’s deep inspiration of the examiner’s hands, each placed lightly over one hemithorax, with thumbs touching beneath the xiphoid at the start of the breath.
An abnormal mass or fullness palpated in the supraclavicular space may be a clue to a neoplasm or an involved lymph node and suggests a convenient location to obtain a biopsy for diagnosis.
Consolidation of the lung, which causes increased transmission of sound, can be detected as fremitus (i.e., as a palpable vibration) over the affected area while the patient repeatedly vocalizes the traditional “one, two, three” as the examiner moves his or her palms systematically over the two hemithoraces. Conversely, impairment of sound transmission, as by a pleural effusion, diminishes vocal fremitus. In some instances, a pleural friction rub is palpable.
 PERCUSSION OF THE CHEST
PERCUSSION OF THE CHEST
Percussion as part of the physical examination follows Auenbrugger sounding of beer barrels to determine their fluid levels. The response to percussion is impaired whenever something other than air-filled lung lies directly beneath the chest wall. Common causes of dullness to percussion are consolidation or atelectasis of the lung, fluid in the pleural space, pleural thickening, and a large mass at the surface of the lung. Widespread hyperresonance may be elicited in emphysema, and circumscribed hyperresonance over a pneumothorax or large bulla.
 AUSCULTATION OF LUNGS
AUSCULTATION OF LUNGS
Ever since the time of Laennec, physicians have applied a stethoscope to the chest in search of sounds of disease.8 Attention is focused on the intensity and quality of the sounds, as well as on the presence of abnormal (often called “adventitious”) lung sounds.9 Other devices have been used to assess sounds generated by breathing.10,11 Web sites providing access to audio files demonstrating normal and adventitious breath sounds are available (e.g., www.easyauscultation.com/lung-sounds.aspx).12
Changes in the Intensity and Duration of Lung Sounds
The generation of lung sounds requires an ability to move air through patent airways. A global decrease in the intensity of breath sounds over the thorax or a hemithorax may be due to a variety of abnormalities: impaired movement of air due to airways disease (e.g., in emphysema), paralysis of a diaphragm, or complete obstruction of a bronchus. A decrease in audible breath sounds may also occur when the transmission of sounds to the chest wall is impaired (e.g., by a pleural effusion, pleural thickening, or a pneumothorax). A bulla gives rise to a more circumscribed diminution in breath sounds. In a patient with COPD, regional variations in breath sounds correspond to the distribution of ventilation. With adequate pressure of the diaphragm of the stethoscope, it is possible to auscultate the lungs as effectively through thin clothing as over bare skin; of course this approach hinders inspection and percussion.13
An abnormal increase in intensity of breath sounds is accompanied by a change in their character (the sounds become either harsh or bronchial). The abnormal sounds are heard over consolidated, atelectatic, or compressed lung as long as the airway to the affected portion of the lung remains patent. Consolidated lung is presumed to act as an acoustic conducting medium that, unlike normal lung, does not attenuate transmission of tracheal sounds to the periphery.
Noting the duration of the inspiratory and expiratory phases of breathing may be useful. Inspiration is normally audible for a longer period, with little, if any, expiratory noise. A prolongation of expiration, often longer than inspiration, is found with obstructed airways.
Changes in the Transmission of Lung Sounds
Changes in voice sounds are often easier to appreciate than changes in breath sounds. Large pleural effusions, pneumothorax, and bronchial occlusion produce distant or inaudible breath sounds. Transmission of voice sounds is enhanced by consolidation, infarction, atelectasis, or compressions of lung tissue. Accompanying the increased transmission is a change in the character of the voice sounds that causes them to be higher pitched and less muffled than normal (bronchophony). When bronchophony is extreme, spoken words assume a nasal or bleating quality (egophony) and the sound “ee” is heard through the stethoscope as “ay.”14 Egophony is most common when consolidated lung and pleural fluid coexist; sometimes it is heard over an uncomplicated lobar pneumonia or pulmonary infarction. Transmission of whispered voice sounds with abnormal clarity (whispered pectoriloquy) has the same significance as bronchophony.
Changes in the Quality of Lung Sounds
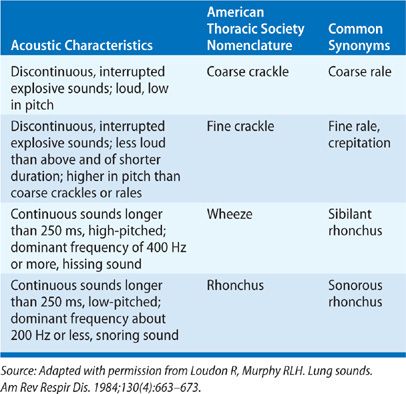
Normal breath sounds have a smooth, soft quality and are described as vesicular. Abnormal, or adventitious, lung sounds have traditionally been resistant to meaningful clinical classification. However, a rational, clinically useful set of definitions based on acoustic analysis of tape recordings and the nomenclature introduced by Forgacs is commonly employed (Table 29-2). Using this approach, lung sounds are categorized as continuous (wheezes, rhonchi, or stridor) or discontinuous (crackles).
TABLE 29-2 Classification of Common Lung Sounds
Wheezes, rhonchi, and stridor are musical adventitious sounds. Wheezes originate in airways narrowed by spasm, thickening of the mucosa, or luminal obstruction. Although wheezes are more apt to occur during forced expiration (which further narrows airways), they may occur during both inspiration and expiration in asthma. Wheezes presumably originate through a combination of limitation to airflow and vibrations in the walls of the airways. Rhonchi are due to the presence of liquid or mucus in the airways; the quality and location may be readily changed by asking the patient to cough, thus moving the secretions. Stridor is predominantly inspiratory and best heard over the neck. Common causes of stridor are a foreign body in the upper intrathoracic airway or esophagus, an acquired lesion of the airway (e.g., carcinoma in adults), or a congenital lesion in children.
Crackles are generally attributed to a rapid succession of explosive openings of small airways that closed prematurely during the previous expiration.15 Crackles have been subdivided according to their timing during inspiration (early or late) and by differences in their quality (“wet” or “dry”); at times they have been termed “rales.” Noting differences in timing has been advocated as a way of distinguishing between possible causes (e.g., “dry” crackles in the fibrosis of interstitial lung disease vs. “wet” crackles in pulmonary edema).16 Unfortunately, wide variation in the interpretation of these sounds generally renders such attempts at classification of little value and often a cause of confusion. Crackles may accompany alterations in the elastic recoil of airways (emphysema), the presence of secretions (bronchitis or pneumonia), inflammation or fibrosis (interstitial lung disease), or fluid (pulmonary edema). Crackles may also be due to atelectasis, as noted in bedridden patients, and may clear with sequential deep breaths.
Pleural Rub
A pleural friction rub is a coarse, grating, or leathery sound that is usually heard late in inspiration and early in expiration; most often a pleural friction rub is audible low in the axilla or over the lung base posteriorly. The rub sounds close to the ear and usually is not altered by coughing.
DYSPNEA
Dyspnea is the medical term for breathlessness or shortness of breath.17 The American Thoracic Society has published a comprehensive discussion of the topic.18 For the patient, dyspnea involves an experience of discomfort in breathing. It is alarming to most and may arouse great concern about a potential dire cause, making it one of the most frequent complaints prompting patients to seek medical evaluation.
In the extensive medical, physiologic, and psychological literature, dyspnea is used variously to designate a variety of sensations, ranging from awareness of breathing on the one hand to respiratory distress on the other. The wide range of meanings is understandable on several counts: (1) dyspnea is a subjective complaint without consistency in objective signs such as tachypnea; (2) few physicians have experienced the respiratory discomfort associated with chest disease, so that most interpretations of the complaint represent extrapolations from normal breathlessness (e.g., after strenuous exercise); (3) most experimental observations relating to dyspnea are based on the study of normal subjects or animals under artificial circumstances; and (4) most physicians apply the term loosely, based on their experience with the predominant patient population that they serve (e.g., patients with COPD or asthma). Despite this variability, in clinical medicine, the complaint of dyspnea almost invariably implies respiratory discomfort.
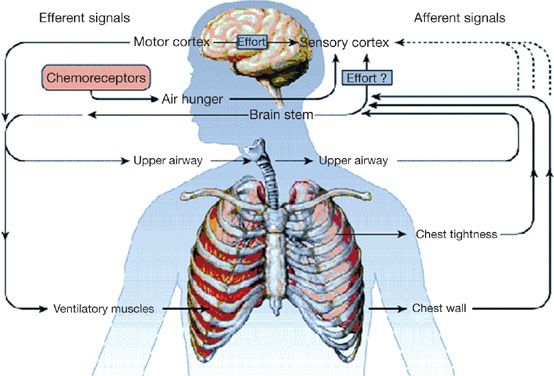
Because of its subjective nature, the sensation of dyspnea is an amalgam of two components. The first is the sensory input to the cerebral cortex, which consists of information from specialized receptors, predominantly mechanoreceptors, at various sites in the respiratory apparatus (predominantly the upper airways) and face (Fig. 29-6). The different sites of stimulation may contribute to disparities in the sensation. Furthermore, no specific area in the central nervous system (CNS) has been identified as the sensory locus for dyspnea. The input – from airways, lungs (via the vagus nerves), respiratory muscles, chest wall, and chemoreceptors – is processed at consecutive levels of the nervous system (i.e., spinal cord and supraspinal regions en route to the sensorimotor cortex). Additional sensory input, triggered by inadequate oxygen delivery or utilization, is poorly understood. The second component is the perception of the sensation, which rests heavily on the interpretation of information arriving at the sensorimotor cortex. The interpretation depends greatly on the psychological makeup of the person.
Figure 29-6 Pathways to the sensation of breathlessness. Respiratory effort is believed to originate as a signal transmitted from the motor cortex simultaneously to the sensory cortex and to the motor command to ventilatory muscles. The brain stem may also contribute to the sense of effort. The perception of air hunger is believed to arise, in part, from increased respiratory activity within the brain stem, whereas the sensation of chest tightness probably results from stimulation of vagal irritant receptors. Although afferent information from airway, lung, and chest-wall receptors most likely passes through the brain stem before reaching the sensory cortex, the dashed lines indicate uncertainty about whether some afferents bypass the brain stem and project directly to the sensory cortex. (Reproduced with permission from Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547–1553.)
A variety of influences may modify the psychological component of dyspnea. During “Kussmaul breathing” (see below), “air hunger” may seem obvious to the observer, even though the patient does not feel short of breath. In contrast, patients with congestive heart failure or COPD frequently volunteer the complaint of “air hunger.” Blunting of the sensorium, as by narcotics or by acute hypercapnia, can eliminate the sensation of breathlessness, even though the abnormal breathing pattern remains. Anxiety may heighten the sense of breathlessness. Indeed, anxiety may be responsible for the clinical syndrome of psychogenic dyspnea, in which the patient experiences “breathing discomfort” that eludes explanation on the basis of a somatic cause. Ill-defined sensations may accompany a full-blown hyperventilation syndrome, consisting of light-headedness, tingling of the hands and feet, tachycardia, inversion of T waves on the electrocardiogram, and even syncope. Breathing discomfort at rest that decreases with activity is often seen when anxiety or other psychological issues are the cause and is a distinctly unusual pattern for dyspnea due to a cardiopulmonary abnormality.
The quality of dyspnea can vary greatly. In normal persons, as well as in those with chest disease, dyspnea may simply signify the transition from an effortless process that is ordinarily conducted at a subconscious level to the awareness that muscular effort is being expended in breathing.19 The healthy athlete completing a sprint experiences breathlessness that can be exhilarating, rather than uncomfortable. The asthmatic often interprets breathlessness in terms of “tightness in the chest.” The patient with COPD often complains of less severe breathlessness than would be expected from the degree of airway obstruction, possibly reflecting adaptation, either to the chronic obstructive airway disease or to CO2 retention.
Patients may use different terms to describe breathing discomfort due to various causes.20–25 In some instances these descriptors may be useful in establishing a differential diagnosis and in assessing the response to therapy.26 Patients with asthma or myocardial ischemia often refer to “chest tightness.”27 Patients with pulmonary edema may suffer a sensation of “air hunger” or “suffocation.”28 Patients with COPD and hyperinflation of the chest often note an inability to take a deep, satisfying breath. Individuals who are deconditioned may complain of “heavy breathing.” Unfortunately, no descriptor has sufficient sensitivity or specificity to be used alone in establishing the cause of a patient’s dyspnea. Ethnic and gender differences in the descriptors and perceptions related to dyspnea have been reported.29,30
 CLINICAL PRESENTATIONS
CLINICAL PRESENTATIONS
Dyspnea may be acute, chronic, or paroxysmal (Table 29-3).
TABLE 29-3 Common Causes of Acute and Chronic Dyspneaa
aMany chronic processes (e.g., left ventricular failure, asthma, and COPD) may have acute exacerbations.
Acute Dyspnea
The usual causes of acute dyspnea in children differ from those in adults. In children, upper airway infection (e.g., epiglottis, laryngitis, or acute laryngotracheobronchitis) is a common cause. In adults, the causes of acute dyspnea are much more varied (Table 29-3). Among the most common are acute left ventricular failure, pulmonary thromboembolism, pneumonia, and spontaneous pneumothorax. Less common, but not unusual, is massive collapse of one lung due to inability to clear the airways of thick, tenacious secretions (e.g., in chronic bronchitis or asthma) or the first attack of asthma.
Chronic Dyspnea
Chronic dyspnea is almost invariably progressive. As a rule, this type of dyspnea begins with breathlessness on exertion—which, in time, progresses to dyspnea at rest. Pulmonologists encounter dyspnea in patients who have COPD; cardiologists more often deal with dyspnea in patients who are in chronic congestive heart failure. Especially in older patients, distinction between the heart and lungs in the etiology of dyspnea, or the relative contributions of each, can be difficult to establish.
Asthma is a common cause of recurrent bouts of dyspnea, which are usually accompanied by cough and wheezing. Cardiac dysfunction is another cause of acute bouts of bronchospasm, especially in middle-aged or elderly persons.
 PHYSIOLOGIC CORRELATES OF DYSPNEA
PHYSIOLOGIC CORRELATES OF DYSPNEA
Historically, attempts to understand the physiologic bases of dyspnea have evolved along four separate lines: ventilatory performance, the mechanics of breathing, chemoreception, and exercise testing. Exercise testing is presented in Chapter 34.
Ventilatory Performance
Early investigations related the sensation of dyspnea to the level of minute ventilation. Dyspnea was found to correlate with excessive minute ventilation relative to the level of oxygen uptake. Most of the increase in ventilation was accounted for by an increase in respiratory rate, especially in patients with stiff lungs. In patients who continued to ventilate excessively for the level of oxygen uptake (e.g., those with chronic left ventricular failure), the sensation of breathlessness gradually diminished, suggesting adaptation to the continued stimulus.
A second ventilatory measurement that proved to correlate well with dyspnea is the maximum voluntary ventilation (MVV). MVV is decreased by diseases of the lungs, airways, or chest wall. The smaller the MVV, the more likely is dyspnea to occur.
A third time-honored approach to measurement is the “breathing reserve.” This value is determined as the difference between the MVV and the actual minute ventilation. In principle, the sensation of breathlessness during the performance of any ventilatory task may be related to the fraction of the maximum breathing capacity (i.e., the MVV) that is used for force generation by the respiratory apparatus. Thus, the closer the minute ventilation is to the maximum breathing capacity, the more likely is the subject to complain of breathlessness. Indeed, when the actual level of ventilation reaches 30% to 40% of the maximum breathing capacity, dyspnea is inevitable. Unfortunately, the breathing reserve correlates better with the dyspnea of normal subjects during exertion than with the dyspnea of chronic bronchitis and COPD or of left ventricular failure. Thus, in COPD the minute ventilation may be a very large fraction of the MVV (>50%) without eliciting dyspnea. In contrast, in acute left ventricular failure, a mild increase in ventilation and a nearly normal MVV may be associated with considerable breathlessness.
Mechanics of Breathing
One teleologic way to regard dyspnea is as a sensation that prompts an unconscious effort to minimize the work, energy cost, or force of breathing. In this light, dyspnea protects the respiratory apparatus from overwork and inefficient operation. This approach has led to exploration of the relationships between dyspnea and the work or oxygen cost of breathing.
Work, Oxygen Cost, and Efficiency of Breathing
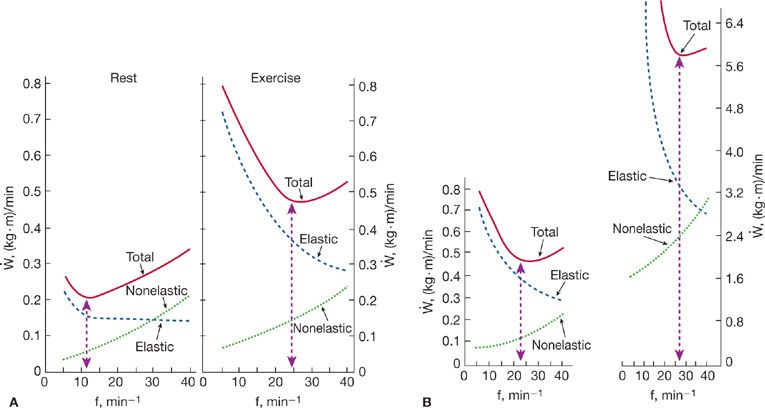
It has not been possible to identify a critical level for the work of breathing at which dyspnea will occur. However, a breakdown of the work of breathing into its elastic, resistive, and inertial components has helped to relate physiologic disturbances to particular diseases. For example, in chronic mitral stenosis with pulmonary congestion, the elastic work is greatly increased (Fig. 29-7), whereas in obstructive airway disease, resistive work predominates. Moreover, such observations have reinforced the concept that patterns of breathing are automatically adjusted to minimize the work done by the respiratory muscles in breathing.
Figure 29-7 Partition of the work of breathing in pulmonary congestion and edema at rest and during exercise. A. Normal. The minimal work of breathing at rest was at a respiratory frequency of 12 breaths/min; during exercise, the minimal work was done at a higher frequency (25 breaths/min). B. Mitral stenosis. At rest, the frequency for least respiratory work was abnormally high (22 breaths/min); during exercise it increased further (to 28 breaths/min). The dashed vertical line (capped by arrowheads) in each frame indicates the respiratory frequency at which respiratory work was minimal. f, respiratory frequency. (Reproduced with permission from Christie RV. Dyspnea in relation to the visco-elastic properties of the lung. Proc R Soc Med. 1953;46(5):381–386.)
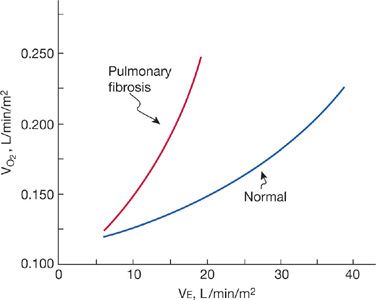
The relationship between ventilation and O2 consumed by the respiratory muscles is curvilinear (Fig. 29-8). This O2 cost of breathing may increase extraordinarily in patients with COPD or with abnormalities of the chest wall. Indeed, in patients with COPD, the quantity of O2 delivered to the respiratory muscles during the large ventilatory effort may fail to satisfy their aerobic needs, leading to anaerobic metabolism and lactic acidosis. Although the greater the O2 cost of breathing the greater the likelihood of dyspnea, the determination of O2 cost provides no more useful insight into the mechanism of dyspnea than does the work of breathing. Calculation of the efficiency of breathing (i.e., the work of breathing related to energy cost) provides no further clarification.
Figure 29-8 Oxygen cost of breathing in restrictive lung disease. Relationship between ventilation and O2 consumption in pulmonary fibrosis. At each level of ventilation, the patient with pulmonary fibrosis does more work and expends more energy in breathing than does the normal subject.
Length–Tension Inappropriateness
The concept of “length–tension inappropriateness” explains dyspnea as a mismatch between the central motor command to the respiratory muscles (i.e., the motor signal emitted from the brain) and the suboptimal (“inappropriate”) shortening of the respiratory muscles elicited by this command (e.g., suboptimal thoracic expansion for any level of central motor command).31 In essence, this concept pictures a decrease, instead of an increase, in the pressure-generating capacity of the respiratory muscles in the face of the increased need arising from the heightened respiratory drive.
Chemoreception
Chemoreceptors in the medulla respond to changes in pH and PaCO2 (see Chapter 11). Peripheral receptors in the aortic arch and carotid body also respond to alterations in PaO2. Acute hypoxia, hypercapnia, and acidosis are the traditional stimuli for ventilation. For example, upon ascent to altitude, acute hypoxia can stimulate ventilation to the level of awareness that may progress to discomfort during exertion. The effects of these stimuli on breathing decrease if they continue unabated. In addition, side effects, such as blunting of the sensorium during chronic CO2 retention, diminish the likelihood of dyspnea, even if the level of ventilation is increased. In patients with abnormal pulmonary mechanics, the onset of abnormalities in blood gas composition, as during exercise, may aggravate or contribute to dyspnea. In general, acute hypercapnia is a stronger stimulus for dyspnea than is acute hypoxia.
 SCALING
SCALING
A variety of scaling methods have been devised to quantify dyspnea during exercise and various experimental settings. Some, such as the Borg Category Scale (Table 29-4), use numbers and descriptive terms to depict a change in the intensity of the stimulus (“threshold stimulus detection methods”). Others rely on visual analog scales, which are straight lines, usually 10 cm long, that extend from “not breathless” at one end to “extremely breathless” at the other. The patient marks on the line the intensity of respiratory discomfort elicited by external stimuli, such as resistive loads or exercise testing. The score is measured as the length of the line between “not breathless” and the mark made by the patient. The Shortness of Breath Scale issued by the American Thoracic Society (Table 29-5) has also been used in one form or another, particularly in epidemiologic studies. A recent method of quantifying dyspnea severity that utilizes patient descriptors appears to be applicable and reproducible in a variety of disorders.32
TABLE 29-4 Modified Borg Category Scale
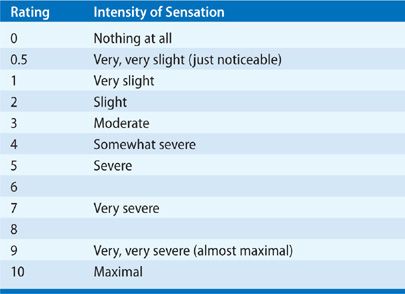
TABLE 29-5 American Thoracic Society Shortness of Breath Scale
DYSPNEA IN OBSTRUCTIVE AND RESTRICTIVE PULMONARY DISORDERS
Two common pathophysiologic categories of pulmonary disease in which dyspnea features prominently are chronic obstructive airway disease and restrictive pulmonary disorders.
 CHRONIC OBSTRUCTIVE AIRWAY DISEASES
CHRONIC OBSTRUCTIVE AIRWAY DISEASES
Several chronic obstructive airway diseases associated with dyspnea are well recognized, including COPD and asthma.
COPD
COPD refers to a spectrum of airway diseases in which obstruction to airflow is the common denominator (Chapters 39 and 40). Cigarette smoking is the leading cause of COPD (Chapter 41). The outer limits of the spectrum are marked by chronic bronchitis at one end and emphysema at the other. Most patients with COPD fall into categories between those limits (i.e., they manifest mixtures of chronic bronchitis and emphysema, which vary in degrees) (Fig. 29-9).
Figure 29-9 Chronic obstructive pulmonary disease (COPD). Sagittal sections showing patterns of emphysema. A. Normal lung from a patient who died of unrelated causes. B. Predominantly centrilobular emphysema. C. Predominantly centrilobular and panlobular emphysema. D. Predominantly panlobular emphysema. Centrilobular emphysema is less marked. The three patients with emphysema (B, C, D) also had clinical manifestations of chronic bronchitis confirmed by histologic sections.
Patients with COPD suffer from disturbances in the mechanics of breathing, abnormal lung volumes, and derangements in gas exchange. The minute ventilation, which may be only slightly increased at rest, constitutes an abnormally large fraction of the maximum breathing capacity (i.e., the “breathing reserve” is low).
Abnormalities in the mechanics of breathing dominate the scene: resistance to airflow is high; the thorax assumes a hyperinflated position, placing the inspiratory muscles at mechanical disadvantage; the work of breathing is greatly increased. The O2 cost of breathing is correspondingly high. Derangements in dead space ventilation and in alveolar–capillary gas exchange add to the afferent stimuli. As a result of the disturbances in mechanics and gas exchange, swings in pleural pressure (a measure of force applied to the lungs) are large, and a considerable muscular effort is expended in breathing; instead of the normal increase of about 1 mL of O2 uptake per liter of ventilation per minute, the O2 uptake increases enormously (up to 25 mL/min). Should O2 delivery to the overworked respiratory muscles be insufficient, fatigue and exhaustion may send nervous and chemical signals of their own to the brain. Finally, if the patient accumulates excess water in the lungs, the juxtacapillary (“J”) receptors provide additional sensory input to the central integrating mechanism. As noted above (see “Length–Tension Inappropriateness”), the convergence of these diverse stimuli upon the sensorimotor cortex may generate an inordinate motor command to the respiratory muscles, which cannot mobilize the thorax sufficiently to generate the pleural pressures needed for adequate ventilation.
One enigma is why patients with COPD maintain different levels of ventilation despite equal abnormalities in conventional pulmonary function tests. The “CO2 retainer,” with respiratory acidosis and arterial hypoxemia, often breathes less than does the non–CO2 retainer in whom blood gas levels are near normal. One teleologic explanation is that the lower ventilation in the CO2 retainer causes less dyspnea. However, this explanation affords no insight into the physiologic mechanism.
Treatment of the patient with COPD is directed at diminishing airways resistance and restoring arterial blood gases toward normal. Unfortunately, bronchodilators generally have only modest effects, and the basic abnormalities in the mechanics of the lungs and airways remain. Consequently, the load on the respiratory muscles is not readily alleviated by medical management. Management strategies also include consideration of ways in which the performance of the respiratory muscles can be improved. These have generally taken the form of training exercises to facilitate adaptive changes and to increase both muscle strength and endurance. Exercise reconditioning in patients with COPD has been shown to diminish breathlessness, possibly owing to three interactive mechanisms: (1) increased mechanical efficiency of the exercising muscles, which decrease ventilatory requirements; (2) improved function of the respiratory muscles; and (3) increased tolerance of the “dyspneagenic” sensory input to the brain. Attempts to rest the respiratory muscles have no lasting effect on dyspnea.
Asthma
Asthma constitutes a different entity, not only in its clinical expressions but also because it is usually episodic and is often related to allergic manifestations, and generally affects younger individuals (Chapters 45 to 47).
The mechanisms described previously for COPD apply as well to asthma. However, these mechanisms do not account for the sensation of “tightness in the chest” or the inordinate sense of labored breathing that accompanies the breathlessness in asthma.
 RESTRICTIVE VENTILATORY DEFECTS
RESTRICTIVE VENTILATORY DEFECTS
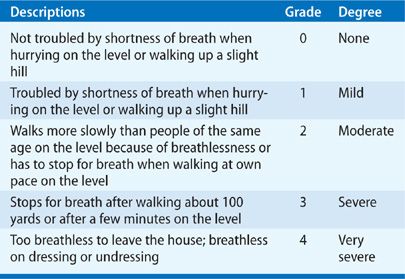
Restrictive ventilatory defects are due to a variety of causes, ranging from lung disorders to diseases that affect the pleural space, as well as neuromuscular diseases that affect the function of the thorax (Table 29-6). Diffuse interstitial disease has many different etiologies and may be either acute or chronic (Table 29-7). Characteristically, in widespread interstitial disease the diffusing capacity is low and is accompanied by a considerable decrease in total lung capacity and in vital capacity, as well as lesser decrements in functional residual capacity and residual volume (see Chapter 33). Similar findings occur in severe kyphoscoliosis or encasement of the lung by pleural thickening (Fig. 29-10). In contrast, in pulmonary vascular disease, such as idiopathic pulmonary arterial hypertension, a low diffusing capacity may be accompanied by normal lung volumes. Neuromuscular disease that affects the inspiratory muscles sufficiently to diminish maximum inspiratory pressures may decrease vital capacity and total lung capacity, leaving functional residual capacity and residual volume increased.
TABLE 29-6 Common Causes of Restrictive Ventilatory Defects
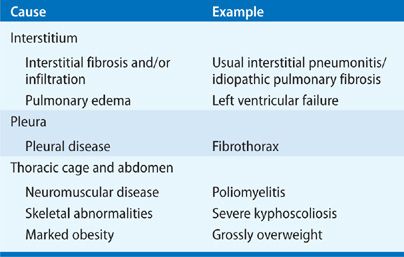
TABLE 29-7 Some Types of Diffuse Interstitial Diseases
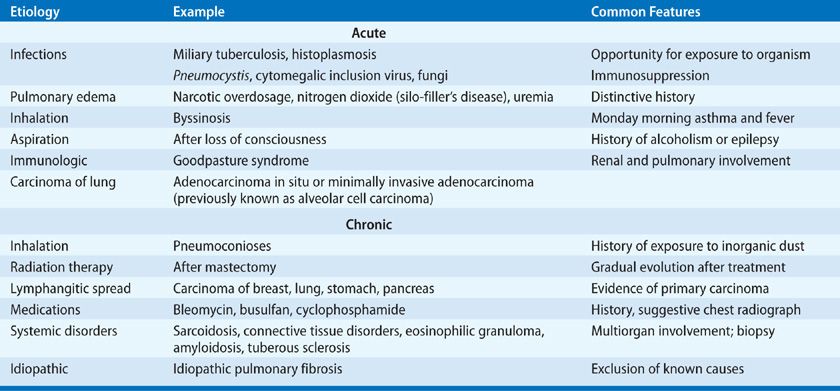
Figure 29-10 Restrictive ventilatory disorders. A. Asbestosis with markedly thickened pleura that encases and compresses the lungs. In addition, the lungs were afflicted with diffuse interstitial fibrosis. B. Compressed, distorted lung in patient with kyphoscoliosis. The lungs were otherwise normal, so that in this instance restriction was imposed by the chest wall rather than by intrapulmonary or pleural disease.
Patients with widespread pulmonary fibrosis breathe faster and maintain a higher minute ventilation than do normal subjects, both at rest and during exercise. The work and oxygen cost of ventilating the stiff lungs are increased. Dyspnea is attributable to the considerable effort by the respiratory muscles in ventilating the stiff lungs and in sustaining the high ventilatory rate. During exercise, dyspnea may become intolerable.
DYSPNEA IN CHRONIC CARDIAC DISEASE
The mechanisms responsible for dyspnea in cardiac disease vary with the extent to which the lungs are stiffened.
Dyspnea occurs in many forms of heart disease that are not associated with congestion of the lungs. Uncomplicated pulmonic stenosis is an excellent example. The symptom is probably related to an inadequate cardiac output during exercise. In Tetralogy of Fallot, dyspnea is sometimes severe and often relieved by assuming a squatting position. In this and other forms of cyanotic heart disease, both dyspnea and fatigue appear during exertion when the arterial oxyhemoglobin saturation decreases appreciably below the resting level.
Cardiac dyspnea is associated with an increase in blood and water content of the lungs. It is a common occurrence in left ventricular failure and mitral stenosis, both of which are accompanied by increases in pulmonary venous and capillary pressures. The engorged pulmonary circulatory bed, coupled with interstitial and alveolar edema, stiffens the lungs (i.e., decreases their compliance) and stimulates the ventilation via “J” receptors. In chronic left ventricular failure, pulmonary fibrosis, consequent to long-standing interstitial edema, contributes to the stiff lungs. Edema of the tracheobronchial mucosa increases airway resistance.
As a result of the stiff lungs and increased airway resistance, swings in pleural pressure during the respiratory cycle are large and the work and energy cost of breathing are increased. Arterial hypoxemia, generally mild, may add to the ventilatory drive. Exercise exaggerates the pulmonary congestion and edema, promotes arterial and mixed venous hypoxemia, and increases the dyspnea.
In patients with pulmonary congestion and edema, tachypnea is a regular feature at rest and increases during exercise. Although tachypnea is consistent, its degree is generally modest and probably not entirely responsible for the dyspnea. Fatigue is a common concomitant of low cardiac output and may stem from diminished O2 delivery to the respiratory muscles, contributing to respiratory discomfort.
 ORTHOPNEA AND OTHER POSITIONAL FORMS OF BREATHLESSNESS
ORTHOPNEA AND OTHER POSITIONAL FORMS OF BREATHLESSNESS
Orthopnea signifies dyspnea in the recumbent, but not in the upright or semiupright, position; it is usually relieved by two or three pillows under the head and back. Platypnea signifies dyspnea induced by assuming the upright position and relieved by recumbency.33
Platypnea may be seen when, due to gravity, increased blood flow worsens right to left shunting of blood through arteriovenous malformations at the lung bases; it may be accompanied by orthodeoxia—desaturation of arterial blood when the patient is upright.
Orthopnea is a hallmark of pulmonary congestion that stiffens the lungs (i.e., decreases their compliance). The decrease in compliance on lying flat is attributable to the fact that more of the lung is located at or below the level of the heart. During recumbency, the swings in pleural pressure, the work of breathing, and the respiratory frequency increase. The increase in respiratory frequency appears to be automatically adjusted to minimize the work of ventilating the more rigid lungs.
Some patients with chronic lung disease or asthma are also intolerant of lying flat. Their discomfort is attributed to the greater difficulty of performing vigorous movements of the chest bellows in the recumbent position.
Finally, patients with asymmetric lung disease may experience trepopnea—dyspnea when the affected side of the chest is in the dependent position, thereby promoting ventilation–perfusion mismatch (Chapter 14) and resultant hypoxemia.
 PAROXYSMAL NOCTURNAL DYSPNEA
PAROXYSMAL NOCTURNAL DYSPNEA
In an episode of paroxysmal nocturnal dyspnea (PND), the patient is aroused from sleep, gasping for air, and must sit up or stand to catch his or her breath; sweating may be profuse. Sometimes the patient opens a room window in an attempt to relieve the oppressive sensation of suffocation. The chest tends to become fixed in the position of forced inspiration. Both inspiratory and expiratory wheezes, often simulating typical asthma, are heard. In some instances, overt pulmonary edema occurs, accompanied by inspiratory crackles. Attacks occasionally recur several times a night, forcing the patient to sleep upright in a chair.
An episode of PND represents precipitous failure of the left ventricle caused by the factors that produce orthopnea (see above), abetted by pulmonary hypervolemia caused by a surge in systemic venous return. Mobilization of peripheral edema from the periphery as the extremities are elevated from the dependent position may contribute to the increase in systemic venous return. The acute increase in pulmonary blood volume increases pulmonary capillary pressures, thereby promoting pulmonary edema, while the surge in venous return imposes an additional burden on the left ventricle.
A variety of factors may trigger an episode of PND: coughing, abdominal distention, the hypercapnic phase of Cheyne–Stokes respiration (see below), a startling noise, or anything that causes a rise in heart rate and further increases the pulmonary capillary and venous pressures. Usually the attack is terminated by assumption of the erect position and a few deep breaths. Cough, an important manifestation of pulmonary congestion, frequently occurs during the attack.
 CARDIAC ASTHMA
CARDIAC ASTHMA
Asthmatic wheezes, often audible in patients with pulmonary congestion, have given rise to the term cardiac asthma. The wheezes are a manifestation of tracheobronchial edema and often are accompanied by overt signs of pulmonary edema. In addition to the reduction in the lumen of the airways and thickening of bronchial walls by edema, the high intrathoracic pressures, which are required to overcome the obstruction during expiration, tend to narrow the airways even further. The resistance to airflow is increased during both inspiration and expiration, and the compliance of the lungs is greatly reduced, reaching values as low as one-tenth of normal. Upon recovery from the acute episode of pulmonary edema, airway resistance and pulmonary compliance return toward normal unless previous episodes have left a residue of pulmonary fibrosis.
DYSPNEA IN ANEMIA
Shortness of breath during exercise or excitement is a common complaint in severe anemia (e.g., hemoglobin concentration under 6–7 g/dL). It is more common in acute than in chronic anemia. Often the dyspnea is associated with dizziness or faintness, and invariably the patient manifests signs of a high cardiac output and low peripheral resistance (i.e., bounding pulse, warm skin, and systolic cardiac murmurs). Although the pathogenesis of the dyspnea is not clear, inadequate oxygen delivery to the respiratory muscles has been proposed.
METABOLIC ABNORMALITIES AND DRUGS
Increases in CO2 production demand a concomitant rise in ventilation to dispose of the metabolic load and, hence, may result in dyspnea. To prevent acidemia, patients with diabetic ketoacidosis may require an enormous increase in minute ventilation in order to reduce PaCO2. Thyrotoxicosis, fever, infection, and pregnancy can also cause an increased minute ventilation, as can drugs, such as aspirin and progesterone.
MISCELLANEOUS DISORDERS
Breathlessness is not uncommon in patients with musculoskeletal disorders. The usual explanation is the heightened motor drive that is needed to activate the weakened respiratory muscles. In the intensive care unit, inadequate ventilator settings for flow and tidal volume may fail to satisfy the intrinsic ventilatory drive of the patient, generating the sensation of breathlessness.
ABNORMAL BREATHING PATTERNS
An important clue to the nature of a clinical problem in pulmonary disease is sometimes provided by bedside observation of a patient’s breathing pattern. The pertinent features are the rate, regularity, depth, and apparent effort being expended in breathing. A normal person at rest breathes about 12 to 15 times per minute, with a tidal volume of 400 to 800 mL. As a result, minute ventilation is normally greater than 5 L/min. The pattern is quite regular except for an occasional slow, deep breath, and the respiratory movements appear effortless.
Severe skeletal deformity, as well as massive obesity, can limit chest excursions to cause alveolar hypoventilation (Chapter 83). Neuromuscular weakness, as in myasthenia gravis or Guillain–Barré syndrome, may do the same, not only by diminishing ventilatory excursions as a result of generalized weakness of the respiratory muscles, but also by causing overload of respiratory muscles (e.g., residual effects of poliomyelitis) (Chapters 83 and 84). Unilateral involvement of one pleural space by pneumothorax, effusion, or fibrothorax limits excursions on the affected side. Massive chest trauma may cause flail chest.
In COPD, a slow respiratory rate and large tidal volumes are characteristic. This pattern presumably serves to minimize the work of breathing. Pursed-lip breathing, a self-induced type of positive-pressure breathing, is often part of the picture. In contrast, persons with restrictive ventilator disorders adopt a breathing pattern that is characterized by small tidal volumes and a rapid respiratory rate, often with little apparent effort. This pattern is seen in patients with a decrease in the distensibility of the lung or chest wall or with reduction of the vital capacity from any other cause. During exercise, minute ventilation increases inordinately with respect to the level of O2 uptake, and respiratory frequency increases more than tidal volume.
Fatigue of the diaphragm and intercostal muscles, sufficient to disturb their coordinated contractions, may give rise to paradoxical breathing, which heralds the onset of respiratory failure.
 CHEYNE–STOKES RESPIRATION
CHEYNE–STOKES RESPIRATION
In the fourth century BC, in a preterminally ill person with fever, sweats, and black urine, Hippocrates described a pattern of breathing in which “the respiration throughout [was] like that of a man correcting himself, and rare and large.” Presumably he had observed Cheyne–Stokes breathing, which was described more graphically by William Stokes two millennia later (in 1854) as follows:
“The symptom in question (previously described by Dr. Cheyne) consists in the occurrence of a series of inspirating, increasing to a maximum, and then declining in force and length, until a state of apparent apnea is established. In this condition the patient may remain for such a length of time as to make his attendants believe that he is dead, when a low inspiration, followed by one more decided, marks the commencement of a new ascending and descending series of inspirations.”
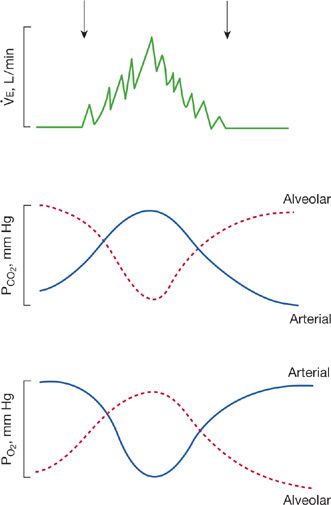
Cheyne–Stokes breathing is characterized by alternating periods of hypoventilation and hyperventilation (Fig. 29-11). In its typical form, an apneic phase, which lasts for 15 to 60 seconds, is followed by a phase during which tidal volume increases with each successive breath to a peak level and then decreases in a progressive fashion to the apneic phase. At the onset of apnea, CO2 tension in brachial or femoral arterial blood is at its lowest. As apnea persists, CO2 tension gradually increases, and respiration is stimulated. CO2 tension continues to increase until maximum hyperventilation is attained, after which ventilation decreases until apnea again occurs. The arterial oxyhemoglobin saturation varies in an inverse manner, being highest at the onset of apnea and lower during midhyperpnea. During the cycle, CO2 tension varies by as much as 14 mm Hg and oxyhemoglobin saturation by as much as 18%.
Figure 29-11 Cheyne–Stokes breathing, illustrating the relationship between the ventilation and the blood and alveolar gas tensions during the periods of apnea and hyperpnea. (Reproduced with permission from Cherniack NS, Fishman AP. Abnormal breathing patterns. Dis Mon. 1975:1–45.)
In patients with congestive heart failure, the respiratory oscillations are attributable to slowing of the circulation so that the blood gases reaching the respiratory centers in the brain are 180 degrees out of phase with those in pulmonary capillary blood. This mechanism has been verified experimentally by eliciting Cheyne–Stokes breathing in dogs by prolonging the circulation time from heart to brain by way of an extracorporeal circuit.
Fluctuations in mental state and electroencephalographic patterns, and evidence of nervous system dysfunction, may occur during Cheyne–Stokes breathing because of swings in cerebral blood flow. In neurologic disorders, Cheyne–Stokes breathing may be due to supramedullary dysfunction, particularly in patients who have destructive lesions in the tegmentum of the pons.
Less common than in heart failure or neurologic disorders is the occurrence of Cheyne–Stokes respiration in normal infants, in healthy elderly persons, and in normal persons at high altitude. It is also seen occasionally after the administration of respiratory depressants (e.g., morphine), often accompanied by an increase in intracranial pressure, uremia, or coma. At one time, the respiratory center was believed to be depressed in Cheyne–Stokes respiration. This hypothesis has been proved to be in error, since it has been shown that the respiratory response to inhalation of CO2 is greater than normal in individuals with Cheyne–Stokes respiration. Respiratory alkalosis is common and the arterial PCO2 remains subnormal in both the apneic and hyperpneic phases.
 KUSSMAUL BREATHING
KUSSMAUL BREATHING
In 1874, Kussmaul described three patients with diabetic ketoacidosis who manifested “air hunger”: they were breathing with large tidal volumes and so rapidly that there was virtually no pause between breaths. In essence, they were breathing at rest as though they were exercising; breathing was accomplished with little apparent effort. Since then, this pattern of breathing has been observed in other types of severe metabolic acidoses (e.g., alcoholic ketoacidosis). The usual sequence leading to this type of breathing is renal failure with a progressive decrease in plasma bicarbonate and resultant acidosis. The “compensatory” increase in ventilation that Kussmaul described mitigates the fall in systemic pH caused by the fall in plasma bicarbonate (see Chapter 17).
 OTHER ABNORMAL PATTERNS
OTHER ABNORMAL PATTERNS
Gasping respirations are characteristic of severe cerebral hypoxia. The pattern consists of irregular, quick inspirations associated with extensions of the neck and followed by a long expiratory pause. It is commonly seen in shock or in other conditions associated with severe reduction in cardiac output.
Hyperventilation is commonly seen in anxious patients without structural disease of the lungs. In some of these patients, striking deep sighs dominate the ventilatory pattern.
DIAGNOSTIC TESTING IN THE EVALUATION OF DYSPNEA
Attention to the history and physical examination findings, as described in the preceding sections, will help to focus the initial approach to diagnosis.34 In most cases, the initial diagnostic impression can be confirmed or excluded with only a few tests, and appropriate therapy instituted or the hunt for a cause continued (Table 29-8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree