Approach to the Patient with Pulmonary Infection
OVERVIEW
Pneumonia is a common cause of infection-related mortality and is one of the most important challenges in clinical medicine. Inappropriate or delayed treatment of pulmonary infection contributes to poor clinical outcomes, avoidable drug exposures, and emergence of antimicrobial resistance. Pneumonia is defined as inflammation of the pulmonary parenchyma caused by an infectious agent. The clinical syndrome of pneumonia may include fever or hypothermia, sweats, rigors, or chills, pulmonary symptoms, such as cough, sputum production, dyspnea, pleurisy, or pulmonary lesions observed on radiographic examination. Nonspecific symptoms are common, including loss of appetite, fatigue, and confusion. The diagnosis and management of pneumonia has been complicated by the recognition of newer pathogens, expanded antimicrobial resistance, increased populations of immunocompromised patients, and by newer diagnostic tools and antimicrobial agents.
Pneumonitis reflects inflammation due to both infection and noninfectious causes. A variety of eponyms have been applied to various forms of pneumonia that may reflect the epidemiology of the process and the likely causative organisms: aspiration pneumonia, community-acquired pneumonia (CAP), nosocomial pneumonia, immunocompromised host, and atypical pneumonia (Table 122-1). These descriptions, coupled with the radiologic appearance and patient-specific epidemiologic factors, are useful in considering empiric therapy while awaiting microbiologic data. These categories may be misleading, emphasizing the importance of obtaining a definitive microbiologic diagnosis in optimizing clinical care (Table 122-2).
Consideration of any unique exposures and potential immune deficits in each host will define the urgency of empiric antimicrobial therapies. Physical findings may also be unreliable—particularly as reliance on radiologic techniques has displaced physical examination as an art form. Dual processes are common (e.g., superinfection of viral illness). Physical findings are often muted in the immunocompromised host. “Crackles” or “rales” are “appreciated” more often than the actual incidence of pulmonary consolidation. Commonly, radiographic appearances are “confused” with etiologic diagnoses: consolidation, bronchopneumonia, miliary patterns, nodules, abscesses, fluid collections, pleural effusions, interstitial pneumonitis, and lymphadenopathy. The goal of the clinician is to distinguish infectious from noninfectious processes, such as heart failure, malignancy, or pulmonary embolism; to initiate appropriate antimicrobial therapy quickly; and to define the microbiology of infectious pulmonary processes to facilitate management and reduce unnecessary exposures to antimicrobial agents.
THE ROLE OF HOST DEFENSES
The presence of pneumonia should be taken as evidence of an immune defect relative to the epidemiologic pressure of the microorganisms (see Chapter 121). Organisms of high native virulence (adhesion, invasive enzymes, motility, intracellular pathogens) may cause infection with a small inoculum size in an immunologically normal host (see Chapter 123). Organisms of low virulence should cause infection only if there is an immune or anatomic predisposition to infection or a high organism burden.1 Microorganisms may reach the lungs via the airways, bloodstream, or lymphatics. Defects in specific components of the immune system (innate and acquired) predispose to specific types of infection (Table 122-3).
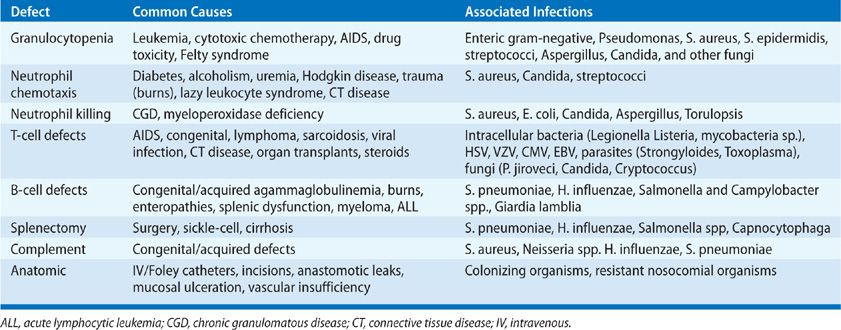
An important first step in many infections is colonization of the upper airway via adhesion of organisms to epithelial surfaces. These surfaces are normally protected by mechanical clearance of organisms via the nose or oropharynx, local production of complement and IgA, saliva, sloughing of epithelial cells, and bacterial interference by “normal flora.” Changes in these surfaces (diminished IgA secretion, changes in production of adhesins, fibronectin, altered lectin binding) predispose to adhesion of microorganisms. Organisms carrying enzymes that can degrade IgA exotoxins, adhesion proteins or pili—are favored in colonizing the respiratory epithelium. Mucociliary clearance may be disrupted by cigarette smoking, or prior infections with viruses, Haemophilus influenzae, or Mycoplasma pneumoniae.
Aspiration may result from altered glottic closure (neurologic injury, sleep apnea, airway intubation, alcohol, anesthesia). Once past the glottis, most bacteria and viruses are small enough (up to 2 μ) to reach the alveoli unless impeded by alveolar lining fluid containing surfactant, IgG, complement, and other proteins. Surfactant includes a variety of components that serve to activate alveolar macrophage and neutrophil functions and may serve as an opsonin (SP-A and SP-D) for many organism types. Organisms surviving the upper airway defenses are left to the cellular components of the lower airways including T- and B-lymphocytes, macrophages, and dendritic cells.
Pulmonary defense mechanisms are also disrupted by systemic infections (sepsis), hyperglycemia and diabetes, acidosis, hypoxemia, pulmonary edema, malnutrition, uremia, age, and lung injury (ARDS). Endotoxin and lipopolysaccharide diminish clearance of bacteria from the lungs. Viral infections may diminish neutrophil and macrophage functions including phagocytosis, chemotaxis, and oxidative metabolism.
GENERAL GUIDELINES IN EVALUATING PATIENTS WITH PNEUMONIA OR OTHER RESPIRATORY INFECTIONS
The individual with pulmonary infection often presents in an ambulatory setting. Evaluation of the patient with possible pneumonia depends on a series of questions that provide clues to management, including the need for hospitalization and selection of antimicrobial agents. Subsequently, microbiologic data provide the basis for adjusting antimicrobial therapy. These initial questions include:
1. Is the process life-threatening? What is the rate of progression of the process? Is it rapidly progressive or gradual? Is there time to delay therapy or diagnostic procedures?
2. Does the patient have immune deficits? Could the process be underestimated based on the absence of normal inflammatory responses (see Chapters 20 and 121)?
3. What are the most common infections in the community or hospital or institution where this “infection” was acquired? In this appraisal, it is helpful to resort to the clinical groupings: community-acquired, nosocomial (hospital, ventilator, healthcare facility), and pneumonia in the immunocompromised patient. Such groupings provide a guide to empiric therapy while evaluation is underway. It is important to understand the incidence of tuberculosis, AIDS, respiratory viral infections, and antimicrobial-resistant organisms (e.g., Pneumococcus or Staphylococcus) in the community and of antimicrobial resistance in the institution. Dual infections are common (e.g., viral plus bacterial) and initial therapy must include both common bacterial and “atypical” pathogens (i.e., Mycoplasma, Chlamydophila, and Legionella species).
4. What are the gross radiologic features of the pulmonary process? Frank pneumonia with or without sputum production, focal infiltrates, lung abscess, chronic cavitary lesion, bronchiectasis, miliary lesions? As a corollary, since pulmonary infections are occasionally generated by the hematogenous rather than the bronchogenic route, consider possible extrapulmonary processes in the pathogenesis of pulmonary infection.
5. For ambulatory patients, does the patient need to be admitted to the hospital for management?2–5 Does the patient have social supports in the community? Can he/she manage oral medications, other therapies, and follow-up visits from home? Does the patient need supplemental oxygen, assisted ventilation, surgery, blood products, monitoring, or isolation?
Initial antimicrobial therapies should be considered to be therapeutic trials—given adequate time to demonstrate effectiveness and subject to revision if ineffective.
 CLINICAL EVALUATION
CLINICAL EVALUATION
The clinical history often suggests the etiology of pulmonary infection. A history of prior infections or underlying clinical conditions (COPD, immune deficits, altered mental status, prior infections) often provides the basis for empiric therapy.
Consider the epidemiologic history (i.e., travel, contacts, exposures, vaccines, medications, prior infections, or hospitalizations): Has the patient traveled or have any hobbies (gardening, hiking, cooking) that might provide an epidemiologic clue (see Table 122-4)?
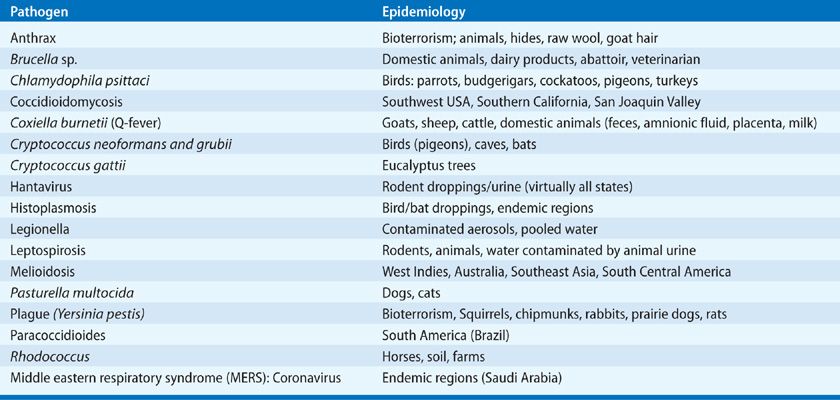
What are the patient’s symptoms: What is the pace of the evolution of respiratory symptoms and of other systemic signs? Prior mild respiratory illness (“the flu”) with improvement and then rapid deterioration is suggestive of bacterial superinfection of viral pneumonitis consistent with Staphylococcus aureus or other bacterial infection, especially in the patient known to have respiratory colonization. Pneumococcal pneumonia may be associated with a single severe rigor with fever and often with symptomatic herpes labialis. The abrupt onset of illness with recurrent (over several days) shaking chills, particularly if associated with mild diarrhea for 1 or 2 days, might suggest Legionnaires’ disease. Gastrointestinal symptoms and confusion may be seen with any infection, but are notable in pneumococcal and Legionnaires’ infections. The differential is broader in immunocompromised hosts with pulmonary-brain (Nocardia species, moulds) or GI-brain (Strongyloides) syndromes.
Skin lesions (e.g., furuncles, endocarditis, or gram-negative sepsis), lymph nodes (symmetrical or regional), retinal examination, ear examination (bullous myringitis with Mycoplasma infection), periodontal disease or absent gag reflexes (with aspiration pneumonia), chest splinting, neurologic disease (pulmonary-brain syndromes) are often ignored but provide valuable clues. The elderly patient with pneumonia will often present with globally depressed mental status while the immunocompromised host may have focal neurologic deficits consistent with embolic or invasive disease. Dullness to percussion, bronchial breath sounds, egophony (“E to A changes”) are suggestive of pulmonary consolidation but may be absent. Patients infected with Pneumocystis jiroveci, Mycoplasma, or viruses (or severe immune compromise) may have a normal chest examination despite abnormal chest radiographs and marked hypoxemia.
Many systemic processes are reflected in abnormalities of blood counts, urinalysis, and routine blood chemistries. Most patients with bacterial pneumonia will have elevated white blood cell counts and increased neutrophils on differential. Viral pneumonia may depress the white cell count. Mixed pictures are common. Mild liver function abnormalities might suggest Q-fever, tularemia, miliary tuberculosis, or Legionnaires’ disease, but are commonly seen with systemic infection. The presence of pigmented casts in the urine and markedly elevated serum levels of creatine phosphokinase might focus attention on the possibilities of influenza virus pneumonia, Legionnaires’ disease, or a pulmonary infiltrate associated with intravenous drug abuse. Rapid screening tests (e.g., for respiratory viruses) are useful, but have limitations in terms of sensitivity.
Clinical measures detect pneumonia in about 79% of cases. Newer assays have been introduced, including measurements of serum procalcitonin (PCT) and C-reactive protein (hsCRP) levels.6 In prospective trials, PCT appears to be sensitive both to the presence and severity of pneumonia and to concomitant bacteremia associated with pneumonia.7 These assays may augment clinical judgment, but are not universally available.
Radiographs allow the practitioner to assess the severity of pneumonia and to distinguish this process form acute bronchitis, which, when infectious, is often viral in etiology (see Chapter 30). No radiographic findings are specific enough to define the microbial origin of a given pneumonia or pulmonary infiltrate. The only definitive way to obtain a specific etiologic diagnosis is through demonstration of the infecting organism—that is, by examination of stained smears of sputum and pleural fluid or other biologic materials, by culture of respiratory secretions and blood, or by demonstration of nucleic acids or proteins from an infecting microorganism, or by demonstrating an increase in antibody titer against the infecting microorganism. Nonetheless, the radiographic picture, taken along with other clinical information, may suggest one or several etiologic agents (see below). Multilobar involvement and the presence of pulmonary effusions are poor prognostic features.
In practice, initial therapy is empiric and based primarily on available clinical clues. The selection of drug(s) for empiric therapy depends on the clinical setting and on the gravity of the pulmonary process. The selection of specific antimicrobial agents is considered in detail in subsequent chapters.
In addition to the decision regarding empiric antibiotics, a key decision in management is whether the patient with suspected pneumonia merits hospitalization (see Chapter 128). In practice, this is often a function of a judgment as to whether appropriate therapy is available in the community with social supports adequate to caring for the patient’s needs (medications, food, comfort) and to bring the patient back for further care if indicated. Multiple semiquantitative methods have been developed to assist in this process.8
The Pneumonia Severity Index (PSI) developed by Fine et al. stratifies patients according to 20 variables and has greater discriminatory power for mortality than the less complex CURB indices.9,10 The “confusion of new onset, urea greater than 7 mmol/L (19 mg/dL), respiratory rate of 30 breaths per minute or greater, and blood pressure less than 90 mm Hg systolic or diastolic blood pressure 60 mm Hg or less with age 65 or older (CURB 65) rely on fewer variables to identify patients at high risk of mortality.10 With one point per variable, risk of death at 30 days increases as the score increases (four points predicts 41.5% hospital mortality).8 Hospital admission is generally advised for those with CURB 65 of two to three points, and ICU admission considered for four to five points.
The Infectious Disease Society of America/American Thoracic Society (IDSA/ATS) Guidelines for Severe Pneumonia and Intensive Care Unit Admission identify the sickest patients, who may ultimately require ventilatory support. The Guidelines can be found online at http://pda.ahrq.gov/clinic/psi/psi.htm and www.mdcalc.com/psi-port-score-pneumonia-severity-index-adult-cap.
RADIOGRAPHIC FEATURES OF PNEUMONIA
No radiologic pattern provides a specific etiologic diagnosis (see also Chapter 30). However, the radiographic pattern, combined with clinical and epidemiologic information, may allow narrowing of the diagnostic considerations while microbiologic data are assembled. Several radiographic patterns may be helpful in categorizing infectious and noninfectious causes: (1) airspace or alveolar pneumonia, (2) broncho- or lobular pneumonia (Fig. 122-1), (3) interstitial pneumonia, and (4) nodular infiltrates. The chest radiographs of a particular patient may not fit neatly into one or another of these categories. Multiple patterns may suggest different etiologies or progression of a disease process. These patterns are generally best defined by CT scanning, which may also detect patterns within a pulmonary process, including lung necrosis; bronchial obstruction (e.g., due to hilar lymphadenopathy or endobronchial tumor); underlying parenchymal disease accounting for pleural effusion, empyema, or loculated fluid collections; bronchiectasis; or fungus balls within cavitary lesions. Subtle changes, including interstitial patterns or infections in immunocompromised hosts (e.g., P. jiroveci), are often better assessed using CT scanning. CT scanning can also assist in determining the best invasive diagnostic approach (needle, BAL, open biopsy) for diagnostic procedures.
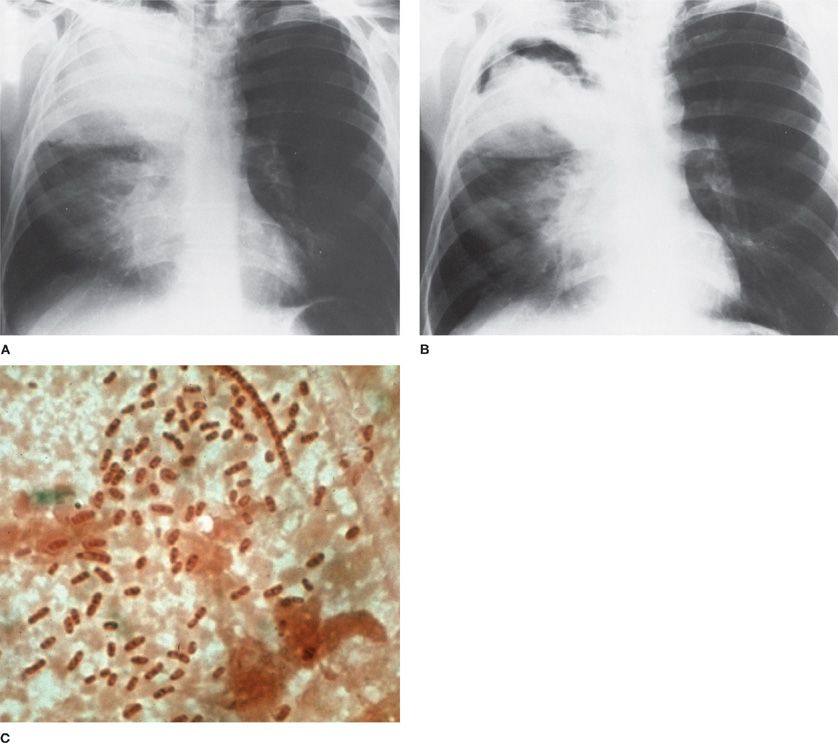
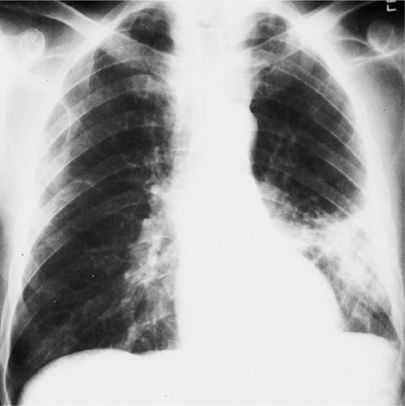
Figure 122-1 A. Dense lobar consolidation involving right upper lobe and right middle lobe in an alcoholic patient with Klebsiella pneumoniae pneumonia. The minor fissure is bulging downward. B. Same patient 7 days later. Despite antibiotic therapy, K. pneumoniae pneumonia progressed to become a necrotic process with formation of multiple abscesses. C. Sputum Gram stain from patient with K. pneumoniae infection reveals gram-negative rod forms with trace of a surrounding capsule. (Used with permission of Dr. R. Greene.)
 PERIPHERAL AIRSPACE CONSOLIDATION: ALVEOLAR AND LOBAR PNEUMONIA
PERIPHERAL AIRSPACE CONSOLIDATION: ALVEOLAR AND LOBAR PNEUMONIA
This form of infiltrate occurs when certain organisms, notably Streptococcus pneumoniae, induce inflammatory edema in peripheral alveoli. When the extent of the consolidation involves an entire lobe, this is the classic lobar pneumonia. Often the process is not that extensive, although the pathogenesis is the same. An air bronchogram is characteristic. Loss of volume is absent or minimal during the acute stage of consolidation, but some atelectasis may develop due to obstruction of bronchi by exudate.
Klebsiella pneumoniae is another common cause of CAP, which, like pneumococcal pneumonia, shows homogeneous parenchymal consolidation containing air bronchograms. Although K. pneumoniae pneumonia classically affects the right upper lobe and produces a dense, homogeneous lobar consolidation with bulging of the fissure, these features are not pathognomonic and cannot be relied on for diagnosis without supportive bacteriologic data (Fig. 122-1). The propensity for K. pneumoniae to produce tissue destruction and abscess formation may result in a shrunken, rather than an expanded, lobe. Pneumococcal pneumonia may also cause bulging of the fissure, albeit less commonly and less prominently. Extensive alveolar consolidation may occur with a variety of other bacterial causes of pneumonia, including mixed anaerobes of aspiration pneumonia and a variety of gram-negative bacilli implicated in nosocomial pneumonias. Occasionally, an unusual configuration of airspace consolidation, spherical pneumonia, occurs, particularly in children, with pneumococcal or H. influenzae pneumonia. It has also been reported with Q-fever (Coxiella burnetti).
In the immunocompromised host, alveolar consolidation on the plain chest radiograph may be delayed and appreciated only by chest CT scan. Among infectious etiologies, common pathogens, such as S. pneumoniae, cause infection in this group of patients and are often of greater severity than in the normal host, often despite less radiologic evidence for infection. Bacterial superinfection of viral processes (e.g., influenza, cytomegalovirus [CMV]) is also common. However, if the consolidation is lobar or multilobar, Legionella pneumophila and M. pneumoniae are common. Other, less likely, infectious agents are fungi (e.g., Aspergillus), Nocardia, and Mycobacterium tuberculosis. Less often, viruses alone (e.g., CMV) elicit a predominantly alveolar pattern. Bilateral diffuse involvement with an airspace pattern resembling pulmonary edema is uncommonly seen in P. jiroveci pneumonia, but may also reflect noninfectious etiologies.
 CENTRILOBULAR AND PERIBRONCHIOLAR OPACITIES: BRONCHOPNEUMONIA
CENTRILOBULAR AND PERIBRONCHIOLAR OPACITIES: BRONCHOPNEUMONIA
In bronchopneumonia, the focus of infection and the inflammatory response is in the walls of the bronchi and surrounding peribronchiolar alveoli, producing a segmental, patchy multifocal distribution. Segmental involvement may become confluent to produce a more homogeneous pattern. Bronchopneumonic patterns are commonly observed in pulmonary infections due to S. aureus or nonencapsulated H. influenzae. With S. aureus infections, macro- and microabscess formation may occur rapidly. Also, pneumatoceles occur during the first week of lung impairment in about half the children with S. aureus pneumonia. These cystic spaces are believed to be the consequence of a check valve opening between a peribronchial abscess and an adjacent bronchus.
A bronchopneumonic pattern of consolidation is commonly observed when CAP follows viral infection or pneumonia is engrafted on underlying bronchiectasis or chronic bronchitis. In such predisposing circumstances, S. pneumoniae infection may produce a bronchopneumonic pattern rather than a lobar consolidation (Fig. 122-2). In the presence of underlying emphysema, the radiographic pattern of pneumococcal pneumonia may also be altered from its usual homogeneous pattern to one that contains multiple radiolucencies (representing unconsolidated emphysematous areas) that may be misinterpreted for abscesses.
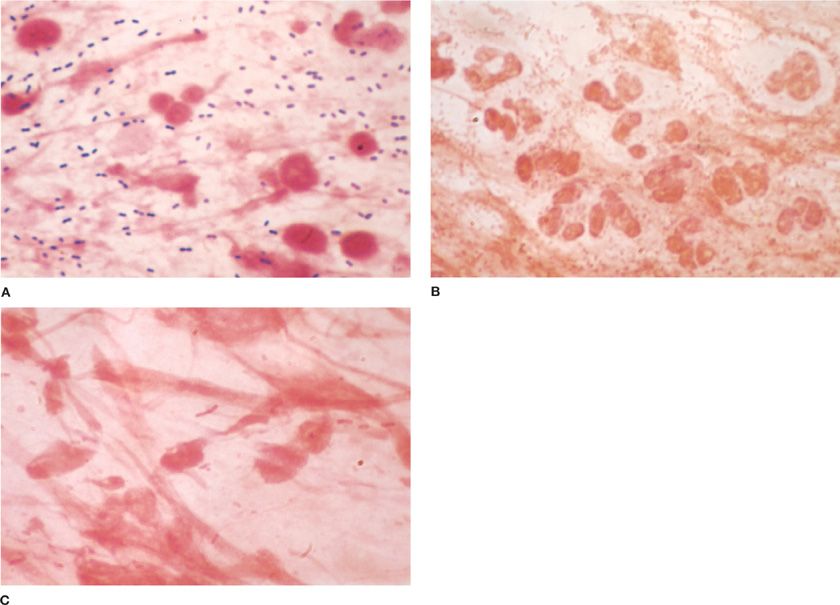
Figure 122-2 A. Gram-stained smear of sputum from patient with pneumococcal lobar pneumonia ×1000. In this field there are numerous gram-positive lancet-shaped diplococci and polymorphonuclear leukocytes. B. Gram-stained smear of sputum from patient with bronchopneumonia superimposed on chronic bronchitis. This field (×1000) is teeming with gram-negative coccobacilli. Many polymorphonuclear leukocytes are present. H. influenzae was isolated from sputum as predominant organism. C. Gram-stained smear of sputum from patient with lobar pneumonia due to K. pneumoniae. In this field (×1000) there are moderate numbers of polymorphonuclear leukocytes and large, thick gram-negative bacilli. (Used with permission of H. Provine.)
Segmental bronchopneumonia is the radiographic picture in pneumonia due to Chlamydophila pneumoniae (Taiwan acute respiratory agent or TWAR), M. pneumoniae, and in many viral pneumonias. Any of the bacterial species that cause nosocomial pneumonia may produce a radiographic pattern of bronchopneumonic consolidation.
Noninfectious etiologies of bronchopneumonia include aspiration of gastric contents, bronchioloalveolar cell carcinoma, granulomatous disease in pneumoconiosis, sarcoidosis, hypersensitivity pneumonitis, bronchiolitis obliterans organizing pneumonia (BOOP), autoimmune diseases, and bronchiolitis obliterans.
 INTERSTITIAL PNEUMONIA (PERIBRONCHOVASCULAR INFILTRATE)
INTERSTITIAL PNEUMONIA (PERIBRONCHOVASCULAR INFILTRATE)
A reticular or reticulonodular pattern of infiltration is the radiographic representation of interstitial inflammation—that is, a peribronchovascular infiltrate. In otherwise healthy persons, M. pneumoniae is high on the list of community-acquired causes of a radiographic pattern of interstitial pneumonia. In some instances, interstitial infiltration progresses to produce patchy consolidation of airspaces, most often in the lower lobes. Pneumonias due to respiratory viruses sometimes have an interstitial pattern that progresses to patchy segmental consolidation or to diffuse airspace disease that resembles pulmonary edema. A variety of noninfectious causes of interstitial lung disease (e.g., hypersensitivity lung disease, collagen vascular disease, and sarcoidosis) may also produce a reticular pattern on the chest radiograph (Fig. 122-3).
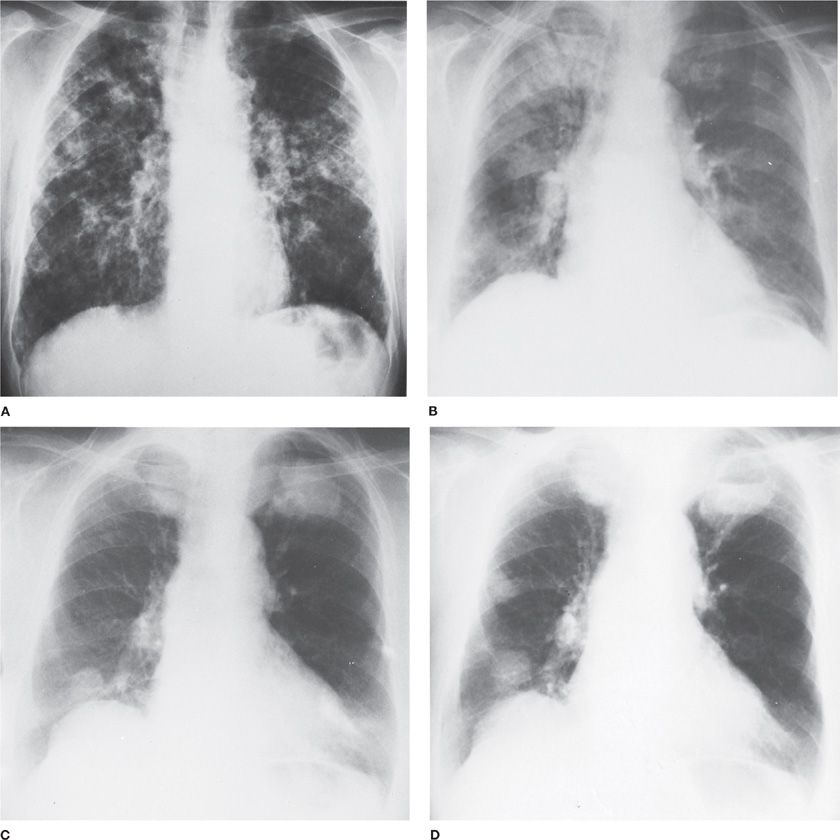
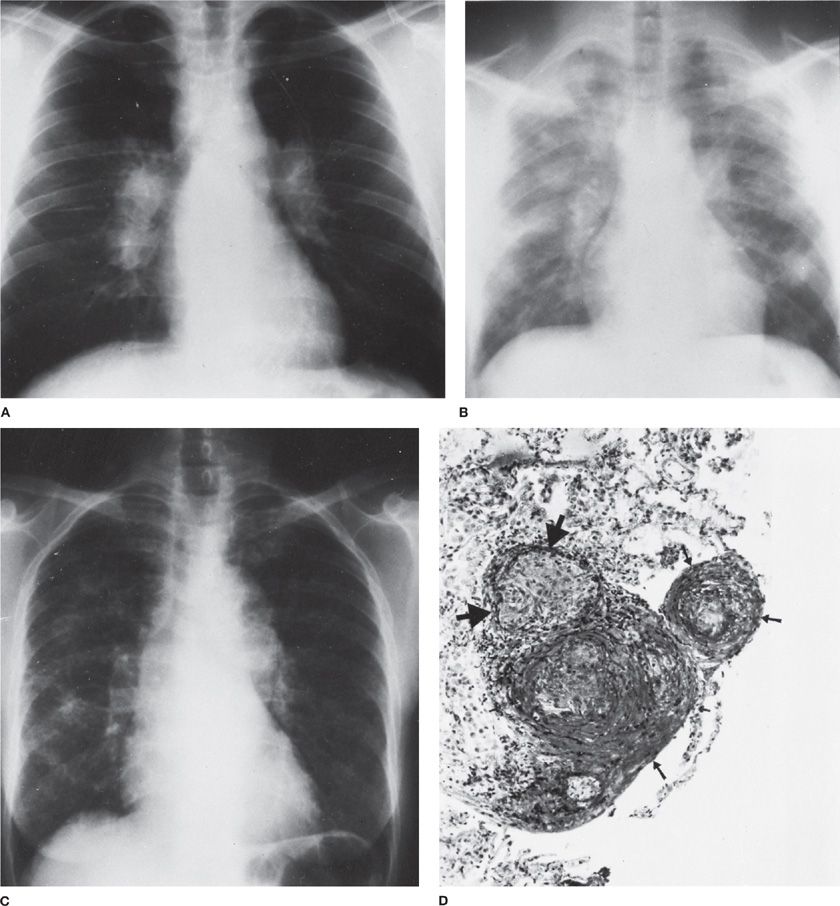
Figure 122-3 Wegener granulomatosis. A. Onset with chills and fever in a previously healthy 64-year-old man. Lung biopsy was interpreted as Wegener granulomatosis. Partial clearing in response to combined chemotherapy (cyclophosphamide and prednisone). B. Onset with malaise, headaches, and fever in a previously healthy 62-year-old woman. Bilateral maxillary sinusitis. Widespread nodular pulmonary infiltrates are most marked on the right. C. Same patient as B after 3 years of intermittent combined chemotherapy. Bilateral large masses. D. Same patient as C, 2 months later. Necrosis within mass in left upper lobe has produced a fluid level.
In immunocompromised patients, particularly in those with AIDS in the absence of effective antiviral therapy, the infectious causes of interstitial pneumonia are broadened to include P. jiroveci pneumonia and opportunistic viral agents (e.g., CMV, varicella-zoster, herpes simplex). Noninfectious causes of a reticular pattern on chest radiography in an immunocompromised host include drug-induced (e.g., bleomycin, methotrexate, or sirolimus) pneumonitis, early radiation pneumonitis, and pulmonary edema.
 NODULAR INFILTRATES: ROUND PNEUMONIA
NODULAR INFILTRATES: ROUND PNEUMONIA
Nodular infiltrates are considered here as well-defined large (greater than 1 cm on the chest radiograph) round focal lesions. Such a lesion may represent small aspirational abscesses (without air–fluid levels), a fungal or tuberculous granuloma, or a lesion of pulmonary nocardiosis. Multiple nodular infiltrates may also represent the necrotic lesions that develop in the lung secondary to the septic vasculitis produced by staphylococcal or pseudomonal bacteremia or the consequences of fungemic spread of candidal infection from an infected intravascular catheter. Infected nodular pulmonary lesions are sometimes caused by septic pulmonary infarcts produced by infected emboli that originate from right-sided bacterial endocarditis, septic thrombophlebitis of pelvic veins, or septic jugular vein phlebitis. On rare occasions, similar nodular lesions are produced by necrotic (but not infected) pulmonary infarctions; primary or metastatic neoplastic lesions may have a similar appearance. Nodular lesions that undergo rapid necrosis with cavity formation can be a feature of granulomatosis with polyangiitis (formerly known as Wegener granulomatosis) (Fig. 122-4).
Figure 122-4 Tuberculous cavities. In each instance, the organisms were seen on smear and identified by culture. A. Cavity amid consolidation in a 56-year-old African American man. B. Bilateral, multiple cavities in a 72-year-old African American man. C. Spread from original involvement of right upper lobe in a 48-year-old African American woman.
In the immunocompromised patient, nodular infiltrates may be due to bacteremic or fungemic spread of infection, most often as a result of nosocomial infection caused by an infected intravenous catheter. In this type of patient, nodular lesions should bring to mind the possibilities of pulmonary nocardial infection, aspergillosis, or other fungal infections. Tuberculous granulomas in the lungs may develop or enlarge in the immunosuppressed patient. Metastatic neoplasm or lymphoma sometimes presents a similar radiologic picture. Multiple small nodules, larger than miliary lesions but smaller than the gross nodular lesions described above, raise the possibility of varicella-zoster or CMV infection of the lung.
 MILIARY AND MICRONODULAR PULMONARY DISEASE
MILIARY AND MICRONODULAR PULMONARY DISEASE
Disseminated miliary lesions of infectious nature suggest tuberculosis, nontuberculous mycobacteria, and endemic fungi (histoplasmosis, or blastomycosis) in either the normal or immunosuppressed host. In the immunosuppressed patient, a miliary pattern may also be seen in disseminated cryptococcal infection or bacteremic spread of bacterial or candidal infection (Figs. 122-5 and 122-6).
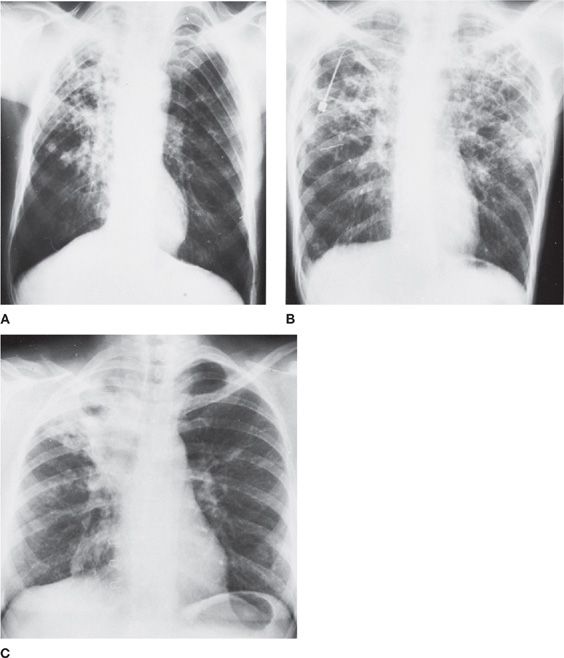
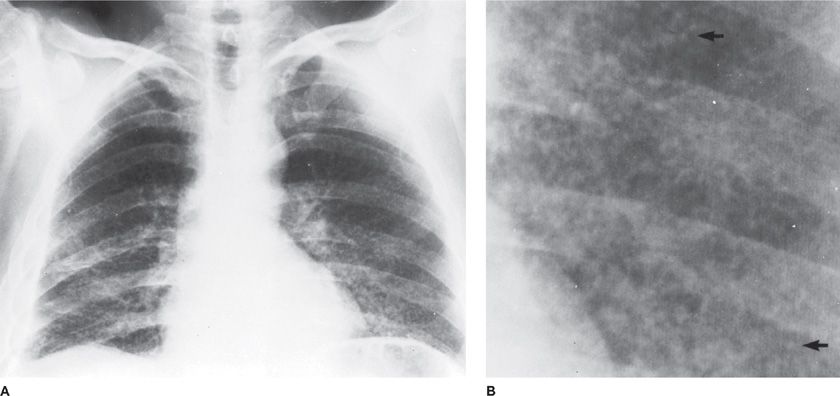
Figure 122-5 Miliary tuberculosis in a 45-year-old immigrant from Portugal with old calcified tuberculous empyema on the right. A. Fine nodularity present in both lungs. B. Arrows point to individual miliary lesions, which are more readily visible with added magnification. (Used with permission of Dr. R. Greene.)
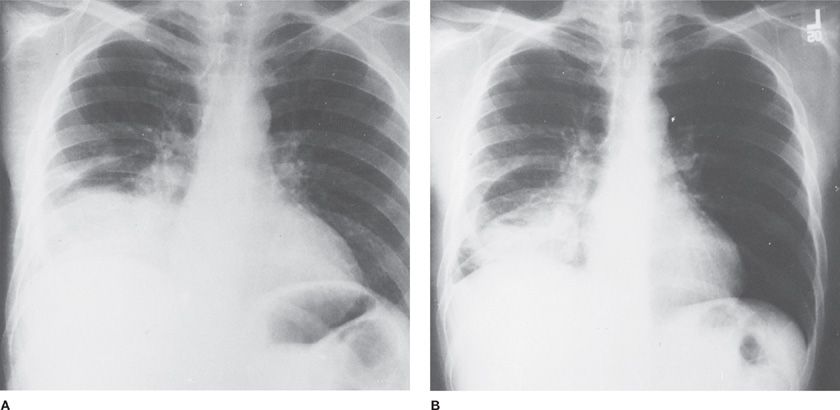
Figure 122-6 Necrotizing pneumonia, probably secondary to aspiration in a 39-year-old man, smoker and drinker, previously healthy. Onset with cough, shortness of breath, fever, and right-sided pleuritic pain. Despite antibiotics, signs and symptoms progressed to include high fevers, night sweats, greenish sputum, leukocytosis, and manifestations of hypertrophic osteoarthropathy. A. On admission, there was consolidation of right lower lobe, a right hilar mass (or adenopathy), and right pleural effusion. Mediastinoscopy and bronchoscopy revealed no tumor. B. Three months later, the process in the right lower lobe is more circumscribed. Right lower lobectomy revealed extensive necrotizing pneumonia, multiple abscesses, and “reactive” lymph nodes. Postoperatively, the patient was free of signs and symptoms, including hypertrophic osteoarthropathy.
 APPLICATION OF RADIOGRAPHIC TECHNIQUES TO THE CLINICAL DIAGNOSIS OF PNEUMONIA
APPLICATION OF RADIOGRAPHIC TECHNIQUES TO THE CLINICAL DIAGNOSIS OF PNEUMONIA
A consistent approach to the incorporation of radiographic imaging in evaluating a patient with suspected pneumonia is helpful. One such approach incorporates the following four steps: (1) Define the radiographic pattern as lobar (Fig. 122-1) or segmental consolidation, patchy bronchopneumonia, nodules (large, small, or military) (Fig. 122-5), or an interstitial process (Table 122-5). Many large round pulmonary densities in a renal transplant recipient suggest Nocardia infection, rather than Pneumocystis pneumonia, whereas in a heroin addict with cough, fever, and pleuritic chest pain, such densities suggest acute right-sided endocarditis rather than pneumococcal pneumonia. (2) Compare the radiograph with prior studies: Is the process old or new? Are there multiple processes? Has the patient had surgery in the intervening period? Is the spleen enlarged or absent? (3) Consider confounding variables: Is it too early in the process to detect radiologic changes (first 18–24 hours)? Is the patient neutropenic (early viral or fungal pneumonitis) or otherwise immunocompromised (P. jiroveci pneumonia with minimal or no findings on plain chest radiographs)? Dehydration is commonly cited as a cause of false-negative chest radiographs—but, in general, this concept is probably over-rated. (4) Consider CT scanning: CT scanning is sensitive to changes unrecognized in plain radiographs and may be useful in guiding invasive procedures.
TABLE 122-5 Radiographic Features and Differential Diagnosis of Pneumonia in the Immunocompetent Host

EXAMINATION OF CLINICAL SPECIMENS AND ESTABLISHMENT OF A MICROBIOLOGIC DIAGNOSIS
The traditional approach to pneumonia places great emphasis on establishing a specific microbiologic diagnosis for pneumonia. An appropriately stained smear of sputum or pleural fluid, bronchoalveolar lavage (BAL) fluids, blood buffy coat, skin lesions, or throat swabs may provide a provisional diagnosis and allow deployment of more targeted antimicrobial therapy. This is discussed in detail below. Examination of sputum samples should not delay treatment.
Guidelines of the IDSA/ATS require administration of antimicrobials within 6 hours of presentation, notably for elderly, critically ill, and immunocompromised individuals.11 However, in practice, the yield of most microbiologic evaluations is poor and the outcomes of therapy for pneumonia not requiring hospitalization quite good.12
Bartlett has observed that sequential clinical series appear to demonstrate a gradual reduction in the frequency with which the Pneumococcus is identified as the etiologic agent of CAP. The prevalence of S. pneumoniae in clinical studies from North America (20%–60%) and Great Britain (60%–75%) and in 4416 patients by Bullowa et al. in 1937 (81%) have gradually dropped to 5% to 12% in more recent studies. In more recent studies, the Pneumococcus has been replaced by viruses, aspiration, and a broad array of bacteria. This observation likely reflects the identification of organisms not identified in early studies by advanced techniques, a reduction in the detection of the Pneumococcus due to successful empiric therapy, shifts in “community flora” by antimicrobial therapies, as well as reduced efforts to obtain a microbiologic diagnosis.
In practice, most patients with pneumonia are treated empirically. In the United States, the performance and interpretation of Gram stain analyses is restricted to licensed laboratory technicians (CLIA88), which makes more difficult the rapid analysis of clinical specimens. The availability of rapid culture–independent microbiologic assays for viral respiratory infections and for some common pathogens (e.g., antigen detection for pneumococcal or Legionella infections, rapid viral screens) has, to some extent, reduced the need for other modalities of diagnosis.13 Further, the availability of an etiologic diagnosis in CAP may not reduce mortality or the length of hospitalization in normal hosts, likely a reflection of improved antimicrobial therapies.14,15 Thus, for community-acquired infection, etiologic diagnosis is often reserved for the sickest patients requiring hospitalization.
Multiple factors may mitigate an effort to obtain an etiologic diagnosis for pneumonia: increasing antimicrobial resistance in the community and in hospitals with the availability of in vitro susceptibility testing; a growing population of immunocompromised hosts in whom appropriate therapy is essential; for the adjustment of therapy when initial therapy fails; to establish the epidemiology of infection (public health); and to support antimicrobial stewardship programs.
 SPECIMEN QUALITY
SPECIMEN QUALITY
A microorganism isolated from deep coughed sputum cultures obtained before the initiation of antimicrobial therapy is considered relevant if detected in respiratory specimens, blood, or both, excluding normal skin or mucosal flora and the specimen is of good quality, that is, >25 leukocytes and <10 epithelial cells per high-power field. Specimens should be plated for cultures within 2 hours or stored at 4°C until plated. In practice, many patients with pneumonia (~30%) cannot produce good sputum samples, many have had prior therapies that alter the success of microbial cultures (15%–30%), and 40% to 60% of cultures are negative.16–19 A specific etiology can be established in less than 50% of cases using routine studies (sputum and serologies). Sputum induction using inhaled nonbacteriostatic saline mist and chest percussion may increase the yield for Pneumocystis, mycobacteria, and malignancy on cytologic examination.
 MICROBIOLOGIC TESTING
MICROBIOLOGIC TESTING
Noninvasive studies may provide information indicating the specific microbial cause of a pulmonary infection or may narrow the field of likely etiologic agents.
The Gram Stain and Point-of-Care Testing
Gram-stained smears provide information not only regarding the morphology and the tinctorial properties of bacteria (and some fungi) but also about the presence of polymorphonuclear leukocytes and squamous epithelial cells, the latter indicating that the specimen originated in the upper, rather than the lower, respiratory tract (Fig. 122-7). Other special staining methods provide additional data including Kinyoun and modified acid-fast stains for mycobacteria, Actinomyces, or Nocardia species, Wright–Giemsa or a variant such as Diff-Quik or direct fluorescent antibody staining of induced sputum samples for P. jiroveci or Legionella species may provide a diagnosis. Calcoflour white and silver stains are available to stain filamentous moulds.
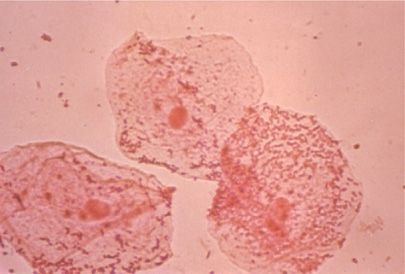
Figure 122-7 Three large oropharyngeal epithelial cells from a specimen of “sputum” that is inadequate for Gram stain analysis and culture because of its origin in the upper respiratory tract. Note the large number of organisms agglutinated on the surface of the squamous epithelial cells (×400).
Culture of sputum or blood or other bodily fluids may provide a specific etiologic diagnosis when evaluation of a sputum smear have not supplied a provisional diagnosis, either because the infecting agent cannot be distinguished from components of the normal upper respiratory tract flora incorporated in the specimen or because the particular microorganism is not visible on Gram-stained smear (e.g., Aspergillus, Mycobacterium, Mycoplasma, or Legionella species).
Cytologic Examination
Examination of Gram-stained sputum smears can be of major value in pinpointing a bacterial cause of pneumonia and guiding initial and subsequent therapy. In practice, therapy is generally initiated prior to the availability of sputum examination. The quality of samples of expectorated or induced sputum for culture and interpretation stained smears can be determined by cytologic examination. Ten or more squamous epithelial cells (normally exfoliated from the oropharynx) per low-power (×100) magnification field indicate that the specimen is unsatisfactory; culture of such a specimen correlates poorly with results from culture of a transtracheal aspirate (Fig. 122-7). The presence of numerous polymorphonuclear neutrophils on Gram-stained smear (10–25 or more per low-power microscopic field) in the absence of an excessive number of squamous cells (see above) is indicative of a good specimen for bacteriologic evaluation.
Examination of Gram-Stained Smears for Bacteria
The oil immersion fields examined, and the immediately adjacent fields should not contain any squamous cells; each should also contain at least three or four neutrophils (in nonneutropenic hosts). The presence of squamous cells not only indicates that the specimen is derived from the upper respiratory tract but also may be confusing to the uninitiated because of the large number of bacteria, often gram-positive diplococci, which might be mistaken for S. pneumoniae, adherent to the surface of these cells.
Multiple bacterial respiratory tract pathogens have characteristic morphologies and strongly suggest an etiologic role when present in a suitable respiratory specimen containing inflammatory cells (in the absence of neutropenia). However, many common organisms are not visualized on stained smears. Such organisms include S. pneumoniae (gram-positive oval or lancet-shaped diplococci), H. influenzae (small, pleomorphic gram-negative bacilli), M. catarrhalis (gram-negative, biscuit-shaped diplococci), or the similar-appearing Neisseria meningitis, enteric gram-negative bacilli (not distinguishable from one another with respect to species except for large encapsulated rods that are suggestive of Klebsiella), and S. aureus (large gram-positive cocci in small groups or clusters) (Fig. 122-8). Since normal oral flora includes a variety of streptococcal species that are morphologically somewhat similar to S. pneumoniae, sputum smears may be misinterpreted. Thus, a predominance of gram-positive diplococci in multiple appropriate oil immersion fields needs to be observed to implicate S. pneumoniae (Fig. 122-9).19 A quantitative aspect to the evaluation has been suggested: at least 10 gram-positive lancet-shaped diplococci per oil immersion field predicts the isolation of S. pneumoniae from sputum cultures with specificity of 85% and sensitivity of 62%.
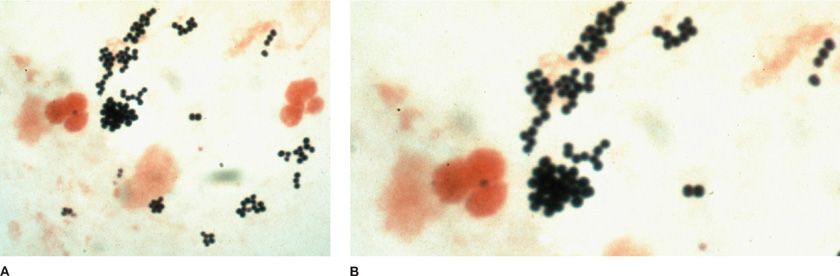
Figure 122-8 A. Left upper lobe pneumonia in a 17-year-old girl due to S. aureus that has progressed to formation of a huge lung abscess. Note air–fluid level. (Used with permission of Dr. R. Greene.) B. S. aureus on Gram-stained smear from a drug addict with right-sided bacterial endocarditis. In this field (×1000) there are polymorphonuclear leukocytes and clusters of gram-positive cocci. (Used with permission of H. Provine.)
Figure 122-9 A–C. Chest radiographs illustrating the various stages of sarcoidosis. A. Stage I, bilateral hilar adenopathy. B. Stage II, bilateral hilar adenopathy with parenchymal infiltrates. C. Stage III, parenchymal infiltrates without hilar adenopathy. D. Transbronchial lung biopsy from a patient with sarcoidosis. Small arrows indicate granuloma with a surrounding rim of collagen (confirmed by positive trichrome staining). The large arrows indicate a granuloma without a surrounding rim of collagen. Original magnification ×10.
Gram-stained smears may be helpful not only in the etiologic diagnosis of community-acquired bacterial pneumonia due to the usual respiratory pathogens, but also in supporting a diagnosis of atypical pneumonia when sputum examinations repeatedly show neither neutrophils nor bacteria. Uncommon bacterial species may be implicated in a pulmonary infection on the basis of unusual morphology on Gram-stained smear: irregularly staining, beaded, delicate gram-positive branching filaments suggest either Nocardia or Actinomyces (Fig. 122-10). Several organisms, uncommon causes of pulmonary infection, have morphologic characteristics that may mimic other, more common respiratory pathogens. Pasteurella multocida and Acinetobacter species, both small gram-negative coccobacilli, have each been mistaken in sputum of patients with pulmonary infections for either H. influenzae or M. catarrhalis.
Figure 122-10 Actinomycosis in a 54-year-old chronic alcoholic man with pyorrheic gums who was admitted with signs of brain tumor. Chest radiograph shows mass in left lower lobe. Computed tomography is consistent with brain metastasis. Transthoracic needle aspirate revealed Actinomyces israelii.
Sputum or pleural fluid with foul odor provides evidence of activity of anaerobic organisms in infective processes such as lung abscess, aspiration pneumonia, empyema, and, occasionally, bronchiectasis. In these settings, the findings on Gram-stained smear may corroborate the preliminary diagnosis. Organisms of the P. melaninogenicus–P. asaccharolyticus group are small gram-negative coccobacilli. Fusobacterium nucleatum is a long, tapering, pale-staining gram-negative bacillus with irregularly staining gram-positive internal granules. Purulent secretions or pus from such anaerobic infections contains numerous neutrophils and usually a mixture of bacterial species, including anaerobic and microaerophilic streptococci on stained smear.
Examination of Ziehl–Neelsen or Fluorochrome-Stained Smears for Mycobacteria
The number of new cases of tuberculosis in the United States steadily declined over past decades, reaching a nadir in 1995. During the period 1985 to 1991, the rate of development of new cases (increasingly due to multidrug-resistant [MDR] strains of M. tuberculosis) increased, primarily associated with microepidemics among the urban poor, racial and ethnic minorities, drug abusers, hospital and correctional facility populations, and patients with HIV infection. TB has decreased in AIDS due to the efficacy of highly active antiretroviral (HAART) therapies.
Pulmonary tuberculosis in the aforementioned settings may take the form of chronic cavitary tuberculosis or forms more likely to suggest pyogenic or atypical pneumonia—that is, progressive primary tuberculosis and tuberculous pneumonia (see Chapter 131). Acid-fast smears of sputum may provide the very first evidence of this disease. Mycobacteria are seen on smears of about 50% of specimens that subsequently prove to contain M. tuberculosis. Most laboratories currently employ a fluorochrome stain with auramine–rhodamine (mycobacteria fluoresce orange-yellow) for initial examination of sputum or other body fluids. Molecular diagnostics (PCR) for M. tuberculosis are also available for direct application to clinical specimens.
Atypical mycobacteria may be demonstrated on sputum smears of patients, usually older people with slowly progressive pulmonary disease or in immunocompromised hosts (see Chapter 132). Disseminated M. avium complex infection in patients with AIDS is usually diagnosed by isolation of the organism from blood culture or by histopathologic diagnosis on biopsy. However, the organism may be demonstrated on acid-fast smears and culture of respiratory secretions even though there may be little radiographic evidence of pulmonary infection directly attributable to its presence. Improved culture techniques are available to more rapidly isolate mycobacteria from clinical specimens. Modified Ziehl–Neelsen–stained smears are helpful in detecting Nocardia.
Fungal Wet Mounts (Potassium Hydroxide, KOH) Preparations
Fungal wet mounts, smears stained with Calcofluor white chemifluorescent agent or phase-contrast microscopy, are employed when epidemiologic considerations suggest community-acquired pulmonary mycoses (particularly coccidioidomycosis and blastomycosis). They should be a routine part of evaluation of respiratory secretions and lung biopsy materials from immunocompromised patients in whom additional fungal pathogens (e.g., Aspergillus and mucormycosis) may be active. In patients with allergic bronchopulmonary aspergillosis, or with the unexpected detection of Aspergillus in sputum, fungal hyphae must be considered in the clinical context of each patient.
Rapid Detection by Direct Immunofluorescent Microscopy
Direct immunofluorescent antibody (DFA) staining may be useful in rapid diagnosis of respiratory tract pathogens. DFA staining reagents for L. pneumophila are commercially available. Their use is not recommended in examination of sputum specimens because of the presence of cross-reacting species (Bacteroides species, Pseudomonas species, Bordetella pertussis) in the upper respiratory tract. However, biopsy specimens of lung (needle, bronchoscopic, or surgical), bronchoscopic aspirates, BAL washings, and pleural fluid samples are suitable for DFA staining for L. pneumophila.
Although a variety of stains (toluidine blue O, methenamine silver, Wright–Giemsa, Diff-Quik, Calcofluor) are useful in identifying P. jiroveci in induced sputa or BAL specimens, or on imprint smears of tissue specimens, the most widely used diagnostic technique utilizes immunofluorescence with monoclonal antibodies against P. jiroveci. Rapid viral diagnosis (RSV, influenza, parainfluenza, adenovirus) by DFA may be applied to specimens from bronchial lavage or brushings or from nasopharyngeal swabs or washings. Anti-B. pertussis DFA may be used on nasopharyngeal aspirate smears in the presumptive diagnosis of pertussis.
DFA may be used to detect viral antigens (adenovirus, influenza A and B, parainfluenza, and RSV, as well as CMV and HSV) in specimens of bronchial brushings, BAL, or nasopharyngeal washings. Enzyme immunoassay may also be used to detect viral antigens in respiratory secretions.
Giemsa and Other Special Stained Smears for the Diagnosis of Pneumocystis Infection
Since P. jiroveci pneumonia is an alveolar process, examination of routinely collected expectorated sputum samples for P. jiroveci is generally not regarded as rewarding in immunosuppressed patients with neoplastic disease or transplant recipients. In patients with AIDS and in others with advanced pneumonia, induced sputum examination for P. jiroveci may be helpful. Otherwise, fiberoptic bronchoscopy and transbronchial biopsy, combined with BAL, provide the highest diagnostic yield.
Sputum induction employing aerosolized, hypertonic saline provides a diagnosis in up to 80% of cases, particularly if coupled with microscopy of antibody-stained cytocentrifuged specimens. Immunofluorescent assays use monoclonal antibodies to P. jiroveci with induced sputum specimens and have a sensitivity of 69% to 92%, compared with that of 28% to 80% for tinctorial stains. Toluidine blue O and methenamine silver stains stain only the cyst (<10% of the organism burden) and not the trophozoite forms of P. jiroveci. Giemsa and Diff-Quik stain trophozoites and intracystic sporozoites. If results of examination of induced sputum are negative and clinical circumstances warrant further attempts at diagnosis, follow-up bronchoscopy with transbronchial biopsy or BAL is performed. The sensitivity of each of these procedures for the diagnosis of P jiroveci pneumonia is over 90%.
Special Microscopic Examinations
Occasionally, in the setting of apparent pulmonary inflammation with features atypical for infection, microscopic examinations using stains other than Gram stain may be indicated. For example, Wright-stained smears may show the presence of eosinophils in allergic pulmonary aspergillosis or other causes of pulmonary infiltrates that are accompanied by eosinophilia. Cytologic examination of exfoliated sputum using Papanicolaou stain may reveal a pulmonary neoplasm. Birefringent calcium oxalate crystals (needlelike in rosettes or arranged like sheaves of wheat) in sputum cytologic specimens have been reported as suggesting pulmonary infection with Aspergillus (aspergilloma and, occasionally, invasive aspergillosis).
In the intubated or tracheotomized patient, whose tracheobronchial secretions commonly contain neutrophils and often some bacteria on Gram-stained smears, it may be difficult to distinguish between colonization and nosocomial pneumonia.20,21 The presence on light microscopy (×400) of characteristic elastin fibers with split ends (in a drop of tracheal aspirate to which a drop of 40% KOH has been added), in the appropriate clinical setting, is a strong indicator of a necrotizing pulmonary infection.
Intense bacteremia sometimes accompanies pulmonary infections, and the etiologic agent may be demonstrable on stained smears of the buffy coat of centrifuged blood: pneumococci have been identified in Gram-stained or Wright–Giemsa–stained smears of buffy coats from splenectomized patients; occasionally, M. avium-intracellulare has been found intracellularly in acid-fast stains of buffy coats from patients with AIDS.
Additional special microscopic examinations may be indicated for immunocompromised patients who have patchy pulmonary infiltrates on the chest radiograph. For example, the presence of the hyperinfection syndrome of strongyloidiasis (often accompanied by Escherichia coli bacteremia) can be established by the finding of filariform larvae in the sputum and in the stool after the latter is suitably prepared by concentration techniques. Although eosinophilia is often present in patients with strongyloidiasis, it may be absent in the hyperinfection syndrome.
 SPUTUM CULTURES
SPUTUM CULTURES
In most patients with the common types of community-acquired and nosocomial bacterial pneumonia, the etiologic diagnosis can be made on the basis of the combined results of a Gram-stained smear of sputum and of proper culture of a suitable exudative portion of a freshly obtained sputum specimen. The criteria for a proper sample of sputum have been noted earlier. Culture generally entails streak dilution on blood agar and McConkey media. Expectorated sputum should not be cultured anaerobically, since contamination with oral anaerobes is inevitable. Blood agar best supports the growth of gram-positive cocci and most gram-negative rods and is especially useful for evaluation of the colony morphology and hemolysis of streptococci. McConkey agar is selective for gram-negative bacteria and is used to determine bacterial ability to ferment lactose (lactose-positive or -negative organisms). Chocolate agar is not used routinely, but allows recovery of H. influenzae and other fastidious organisms. Because patients with Legionnaires’ disease often have little sputum production and growth requires special media, most attempts to isolate Legionella resort to specimens obtained either by induced sputum samples, fiberoptic bronchoscopy, or lung biopsy or at thoracentesis. Cultures of such materials are plated on buffered charcoal-yeast extract (BCYE) agar. Occasionally, Legionella species can be isolated from sputum with the use of a semiselective medium, either BCYE or BCYE-containing cefamandole, polymyxin B, and anisomycin. Culture is the most definitive method for diagnosis of Legionella infection. Unfortunately, it may take 5 or more days for colonies to appear.
Cultures for mycobacteria are undertaken when clinical circumstances raise the possibility of pulmonary infections due to M. tuberculosis or atypical mycobacteria. Similarly, cultures of sputum for primary invasive mycotic agents (e.g., Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides immitis) are dictated by clinical and epidemiologic circumstances. In immunosuppressed patients, cultures of sputum are also directed toward a variety of opportunistic fungi, including Cryptococcus neoformans, Aspergillus species, and Mucoraceae.
Most hospitals do not have facilities for isolating viruses by tissue culture. This lack poses little problem in dealing with most community-acquired viral pneumonias, for which viral isolation is not necessary and the cost is prohibitive. However, viral isolation from throat washings is warranted in certain circumstances (e.g., to prove the presence of an outbreak of influenza) and to identify a specific viral agent, such as an adenovirus or SARS, as the cause of a serious pneumonia that is not responding to antibacterial therapy. Cultures may be grown in cell lines susceptible to the viral infections under consideration in either standard “tube cultures” or “shell vial” cultures (rapid culture achieved by centrifugation of specimens against the cultured cells). Viral replication in the tissue culture can be confirmed within 48 hours after inoculation with the use of fluorescent monoclonal antibodies. Because CMV and herpes simplex are frequently present in the oral secretions of immunosuppressed patients, isolation of these viruses is apt to be meaningful only if the materials used for the isolation procedure were obtained either by bronchoscopy with protected specimen brush (PSB) or with BAL, lung biopsy, or transtracheal aspiration. In practice, diagnostic cultures (e.g., for CMV, other herpesviruses, and adenovirus) have been replaced by quantitative molecular amplification assays (nucleic acid tests, NAT, polymerase chain reaction, PCR) or antigen detection systems (Table 122-6) that are more rapid and cost efficient than culture systems (see below).