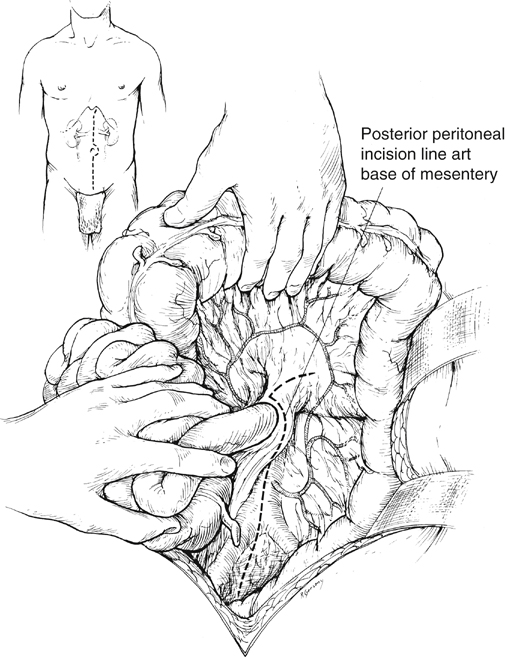
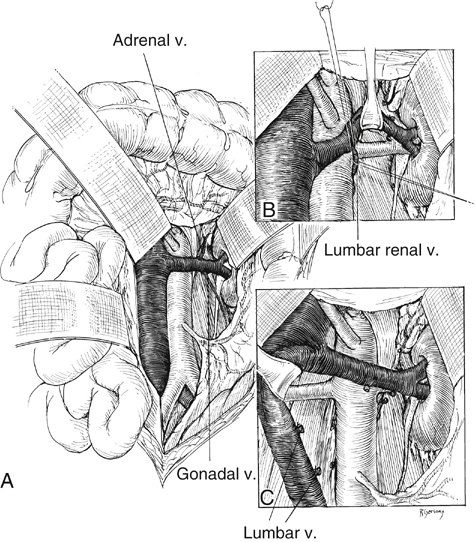
A surgical strategy has evolved for open renal artery reconstruction (Box 1). Hypertension is a prerequisite for renal artery intervention by any method. Management of most unilateral lesions is guided by results of functional studies. Empirical renal artery repair is often performed without functional studies in patients with severe hypertension, especially when hypertension is associated with renal insufficiency and bilateral renal artery lesions. Prophylactic renal artery repair in the absence of hypertension is not recommended, either as an isolated procedure or combined with aortic reconstruction. When the midline xiphoid-to-pubis incision is used, the posterior peritoneum overlying the aorta is incised longitudinally, and the duodenum is mobilized at the ligament of Treitz (Figure 1). During this maneuver, it is important to identify mesenteric arterial collaterals that course at this level. Finally, the duodenum is reflected to the patient’s right to expose the left renal artery. By extending the posterior peritoneal incision to the left along the inferior border of the pancreas, an avascular plane posterior to the pancreas can be entered (see Figure 1) to expose the entire renal hilum. This exposure is of special significance when distal renal artery lesions need to be managed (Figure 2A). The left renal artery lies posterior to the left renal vein. In some instances, the vein can be retracted cephalad to expose the artery; in others, caudal retraction of the vein provides better access. Usually, the gonadal and adrenal veins, which enter the left renal vein, must be ligated and divided to facilitate exposure of the distal artery. Often, a lumbar vein enters the posterior wall of the left renal vein and can be easily avulsed unless special care is taken while mobilizing the renal vein (Figure 2B). The proximal portion of the right renal artery is exposed through the base of the mesentery by ligating two or more pairs of lumbar veins and retracting the left renal vein cephalad and the vena cava to the patient’s right (Figure 2C).
Aortorenal Bypass for Renovascular Hypertension in Adults
Operative Management
Surgical Strategy
Operative Exposure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aortorenal Bypass for Renovascular Hypertension in Adults
