Chapter 112
Aortoiliac Disease
Endovascular Treatment
Richard J. Powell, Eva M. Rzucidlo
As endovascular techniques develop, it is crucial for vascular surgeons to keep apprised of the latest approaches to treating aortoiliac occlusive disease, bearing in mind that treatment should be tailored to the individual patient’s symptoms and comorbidities, as well as the location and extent of disease. This chapter highlights the most recent developments in the endovascular management of aortoiliac occlusive disease.
Background
Progress in endovascular surgery has resulted in a continued shift in the treatment of patients with aortoiliac occlusive disease to less invasive forms of therapy. In the early stages of development, surgeons viewed endovascular approaches to the treatment of aortoiliac occlusive disease with suspicion. In many respects, this was justified because of relatively high complication rates and poor durability. However, early pioneers such as Dotter and Gruntzig persisted and moved the field forward.1 Because of their early work in the development of balloon angioplasty systems and subsequent work in stent development by Palmaz et al, these techniques slowly gained traction in the treatment of patients with peripheral vascular disease.2–4 As improvements in technology such as higher-resolution imaging, lower profile systems, premounted balloon-expandable stents, self-expanding stents, and more recently, stent-grafts have resulted in better outcomes, vascular surgeons now incorporate these treatment modalities into their practices. Today, the majority of patients with aortoiliac occlusive disease can be safely treated with percutaneous endovascular procedures. As the technology further evolves, it is likely that even patients with more advanced aortoiliac occlusive disease will be candidates for endovascular therapy by means of stent-grafts and hybrid open-endovascular approaches.
Indications
There are both patient- and lesion-specific indications for aortoiliac intervention. Patient-specific indications for treatment include lifestyle-limiting claudication, rest pain, and tissue loss. Less frequent indications include vasculogenic impotence and atheroembolization to the lower extremities. As with all vascular reconstructions, the expected benefits of the proposed procedure must be weighed against its potential risks in view of patient comorbidities.
Limb Ischemia
Patients with hip, buttock, thigh, or calf claudication constitute the largest group of patients who undergo aortoiliac endovascular revascularization. Patients with critical limb ischemia (CLI) manifesting as either rest pain or tissue loss frequently have multilevel occlusive disease. In this patient population, the aortoiliac disease is commonly diffuse and complex, often extending into the common femoral arteries (CFAs) and associated with infrainguinal occlusive disease. Patients in whom CFA occlusive disease does not cause significant obstruction can typically be treated with percutaneous approaches that improve perfusion to the lower extremities sufficiently to resolve the rest pain or heal ischemic ulceration. In patients with a significant CFA disease burden, a hybrid approach combining femoral artery endarterectomy and patch angioplasty with simultaneous aortoiliac stenting or stent-grafting often provides adequate perfusion to treat CLI.
Younger Patients
Patients younger than 50 years have worse outcomes following aortoiliac endovascular therapy. However, the same is generally true for open surgical approaches such as aortobifemoral bypass.5–8 Many younger patients cannot be absent from work for the 6- to 8-week recovery period required after open aortic surgery, and as a result, they often opt for the less invasive endovascular approach. In addition, many men are concerned about the relatively high incidence of erectile dysfunction that can occur following open aortobifemoral bypass surgery and thus choose an endovascular approach.
Embolization
Less frequently encountered are patients who present with spontaneous atheroembolization to the lower extremity, or so-called blue toe syndrome, who may benefit from endovascular therapy. This is a controversial indication for endovascular therapy and usually involves placement of a bare or covered stent to trap the underlying pathogenic atherosclerotic lesion and prevent embolization during treatment.9–11 Endovascular intervention is typically not indicated for patients whose atheroembolization is a result of arterial catheterization procedures. In the absence of subsequent catheterization procedures, the risk of recurrent embolization is low.
Improving Inflow for Concomitant Procedures
Endovascular therapy for aortoiliac disease is frequently required as an adjunct to various open infrainguinal procedures to provide adequate inflow. It may be performed simultaneously with lower extremity bypass or in conjunction with femoro-femoral bypass. Indications for treatment in this setting include a resting systolic pressure gradient proximal to the intended infrainguinal bypass procedure of greater than 10 mm Hg or a vasodilator-enhanced gradient of greater than 20 mm Hg.
Contraindications
There are no absolute contraindications to the endovascular treatment of aortoiliac occlusive disease. Relative contraindications are largely anatomic and include juxtarenal aortic occlusion, circumferential heavy (>1 mm) calcification, hypoplastic aortic syndrome, and juxtaposition to aneurysmal disease. Renal insufficiency is also a relative contraindication owing to potential contrast-induced nephropathy, although preventive regimens, minimal contrast techniques, and use of carbon dioxide have reduced the impact of this complication.12–14
Relevant Anatomy: TASC Classification
Lesion-specific indications for the endovascular therapy of aortoiliac occlusive disease can be guided by the Trans-Atlantic Inter-Society Consensus (TASC) guidelines. The TASC classification system (Fig. 112-1) was revised in 2007 to offer more current guidelines on the use of endovascular therapy based on lesion anatomy.15 In general, endovascular therapy is the recommended first-line therapy for TASC A and B lesions and increasingly for TASC C lesions as endovascular techniques improve. Good-risk patients with TASC type C disease can also be treated with open surgery, depending on patient preference. Surgery is usually recommended for TASC D lesions, but advanced endovascular approaches are now being applied to these lesions as well, with good results.16 High-risk patients with TASC C and D disease, CLI, and advanced comorbidities such as severe chronic obstructive pulmonary disease, unreconstructable coronary artery disease, or a low cardiac ejection fraction may be treated with endovascular therapy, acknowledging that this approach will be less durable than open surgical options but likely less morbid.
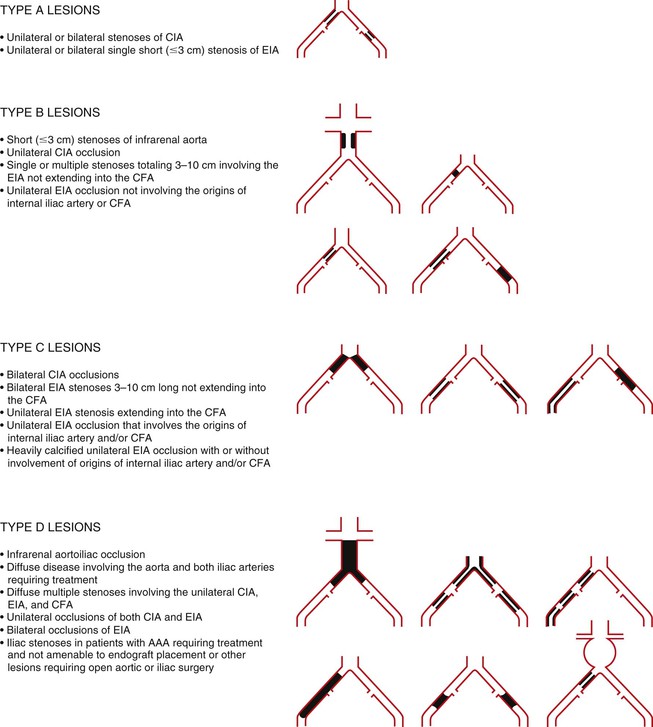
Figure 112-1 TASC classification of aortoiliac lesions. AAA, Abdominal aortic aneurysm; CFA, common femoral artery; CIA, common iliac artery; EIA, external iliac artery. (Redrawn from Norgren L, et al: TASC II Working Group, Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45(Suppl S):S5-S7, 2007.)
Patient Evaluation and Operative Planning
Primary Evaluation
Once a decision has been made that intervention is indicated (see Chapter 109), information must be gathered to determine the location and extent of the atherosclerotic occlusive disease. This begins with a history and physical examination to determine whether the aortoiliac segment is involved. A history of hip, thigh, or buttock claudication; impotence; the presence of a lower quadrant abdominal or CFA bruit; and diminished femoral pulses are all suggestive of aortoiliac occlusive disease. Patients should undergo noninvasive physiologic arterial studies, such as ankle-brachial index (ABI) and toe pressure measurements, if indicated. In patients with a history suggestive of vasculogenic claudication but a normal pulse examination or resting ABI, treadmill testing may help differentiate vascular from neurogenic symptoms.
Noninvasive Imaging
Just as when planning for an open aortobifemoral bypass, additional diagnostic studies are indicated before endovascular intervention to assess the location and extent of arterial occlusive disease and the degree of calcification. The use of arteriography as a purely diagnostic tool is infrequently indicated because of the widespread availability of less invasive imaging modalities that provide anatomic detail without the risks of an invasive procedure. A decision to proceed with endovascular treatment versus open revascularization can be made without the use of arteriography. Imaging modalities currently used to evaluate the aortoiliac segment include duplex arterial mapping, magnetic resonance angiography (MRA), and computed tomographic angiography (CTA). All these modalities have benefits and drawbacks.
Duplex Arterial Mapping
Duplex arterial mapping of the aortoiliac segment and CFAs can adequately assess the location of hemodynamically significant lesions (Fig. 112-2). It is particularly helpful to assess the burden of disease in the CFA, which may potentially alter a planned percutaneous approach through the CFA to an open CFA endarterectomy and concomitant iliac stent/stent graft placement. This modality is especially useful in patients with renal insufficiency who are at risk for contrast-induced renal dysfunction.17 Drawbacks of duplex arterial mapping are that it provides only a semiquantitative assessment of the degree of iliac calcification, it may not adequately image the iliac system in certain patients owing to overlying bowel gas or body habitus, and it requires a significant time commitment and a highly trained vascular technologist.18,19 These constraints have prevented duplex arterial mapping from becoming a more widely adopted imaging modality.
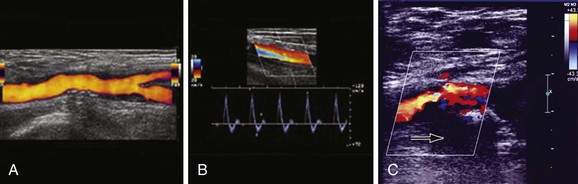
Figure 112-2 Duplex mapping of the common femoral artery. A, Longitudinal view of the common femoral artery (power imaging) and the bifurcation of the superficial and deep femoral arteries. B, Doppler spectral waveform from a normal right femoral artery. The Doppler signal was obtained from a longitudinal view with the Doppler sample volume placed in the middle of the lumen. The characteristic triphasic Doppler signal shows a brisk upstroke to peak systole, reversal of blood flow during early diastole, and a forward flow component during late diastole. C, Longitudinal view of the common femoral artery with greater than 50% stenosis due to calcified posterior plaque (note prominent acoustic shadowing deep to calcified plaque, arrow).
Magnetic Resonance Angiography
MRA can reliably assess the aortoiliac arterial segment, although there is institutional variability in its accuracy. The major drawback to MRA is its failure to provide an accurate assessment of the degree of calcification of aortoiliac lesions.
Computed Tomographic Angiography
The presence of severe calcification has significant implications for operative or interventional planning. Circumferential calcification thicker than 1 mm is a relative contraindication to aortoiliac angioplasty and stenting because of the potential risk of fracture and rupture of the artery. In patients with significant co-morbidities in whom intervention is mandated by CLI, use of a stent-graft may be considered in an attempt to limit the incidence of clinically significant arterial perforation. In this setting, CTA has been used with success to assess calcification of the aortoiliac segment before intervention. The major disadvantages of this imaging modality include exposure to ionizing radiation and the need for iodinated contrast agent, with the risk of contrast-induced renal dysfunction. In appropriate patients, CTA can accurately assess lesion location and extent and degree of calcification. Based on CTA, the severity of aortoiliac and femoral disease can be classified according to TASC; this additional information regarding anatomy and severity of calcification allows the formulation of an appropriate procedural plan and the selection of either open or endovascular therapy.
Evaluation for Common Femoral Artery Disease
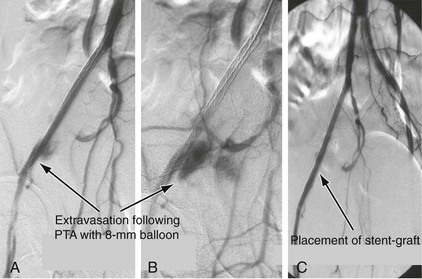
The determination whether to proceed with percutaneous endovascular therapy or an open femoral approach is based on the presence of significant CFA disease. Patients with greater than 50% CFA stenosis on duplex arterial mapping, MRA, or CTA are usually treated with a hybrid approach that entails open femoral endarterectomy, patch angioplasty, and simultaneous stent or stent-graft placement. In general, stent-grafts are used in conjunction with open endarterectomy, especially in the presence of calcific external iliac artery disease due to the risk of arterial rupture and bleeding with bare metal stents (Fig. 112-3). In patients with less severe CFA disease, a percutaneous approach can be undertaken.
Concomitant Aortic Disease
Aortic angioplasty and stenting for lesions proximal to the aortic bifurcation warrant special consideration because these lesions are frequently exophytic and calcific. When treating such lesions, emphasis should be placed on obtaining an adequate hemodynamic response; acceptance of an imperfect angiographic completion image is critical to avoid aortic rupture. Because of the exophytic nature of these lesions, primary stent placement to trap potential atheroembolic debris should be considered. Primary stenting should also be considered for lesions suspected of being the source of atheroembolization to minimize the potential for embolic complications.
Technique
Pretreatment Considerations
Before the intervention, the patient’s history and all pertinent imaging studies are reviewed, and informed consent is obtained. Laboratory tests of coagulation status and renal function are essential.
Avoiding Contrast Nephropathy
Contrast-induced nephropathy (CIN) is defined as an increase in serum creatinine greater than 25%, or >0.5 mg/dL (44.2 µmol/L) within 3 days of intravascular contrast administration in the absence of an alternative cause. Serum creatinine usually returns to baseline within 14 days, however some patients may progress to acute kidney failure requiring dialysis.19a Contrast induced nephropathy is the third most common cause of new-onset acute renal failure in hospitalized patients.20 The incidence may be as high as 25% in patients with preexisting renal impairment or certain risk factors such as diabetes, congestive heart failure, advanced age, and concurrent administration of nephrotoxic drugs.21 For those patients that require dialysis, the median 2-year survival is 19%.21a The single most important risk factor for contrast-induced nephropathy is chronic kidney disease which increases the risk by more than 20 times.21b It is generally accepted that risk of CIN in patient with estimated glomerular filtration rate (eGFR) ≥60 mL/min is extremely low. Studies have suggested that a threshold for CIN exists for patients with eGFR 40-45 mL/min and significant concern should be raised in patients with severe renal dysfunction with eGFR <30 mL/min.21c Patients with both renal impairment and diabetes have up to a 50% risk of developing CIN.21d The majority of studies regarding CIN have been for patients having intra-arterial contrast administration, in particular cardiac interventions. It has been assumed that intravenous (IV) contrast administration has the same risk as intra-arterial injection; however, this assumption is being challenged in recent studies.21c,21e-g Several trials in patients receiving IV contrast have shown a low risk of CIN in patients with eGFR >40 mL/min. Among patients that did develop CIN, none progressed to dialysis and none had fatal outcomes.21h,21i It has thus been proposed that for patients with eGFR <60 mL/min who are receiving intra-arterial contrast, full preventive measures should be initiated. On the other hand, for patients receiving IV contrast, preventive measures are recommended for eGFR <45 mL/min. Preprocedural volume loading and the use of low-osmolar or iso-osmolar contrast agents can decrease the risk of contrast-induced nephropathy.22–24 However, the risk is not eliminated in some patients, even when an iso-osmolar contrast agent is used.25,26 The risk of CIN strongly correlates with contrast volume. Contrast volume in excess of 5 mL/kg strongly predicts CIN requiring dialysis.26a A second contrast dose within 48 hours also significantly increases the risk of CIN.26b-d Because free radicals are postulated to mediate contrast-induced nephropathy,27 alkalinizing renal tubular fluid with sodium bicarbonate28 has been shown to reduce injury. Merten et al reported a single-center randomized controlled trial comparing the infusion of sodium chloride versus sodium bicarbonate as the hydration fluid to prevent renal failure in patients with stable renal insufficiency undergoing diagnostic or interventional procedures requiring radiographic contrast agent.14 The absolute risk reduction of contrast-induced nephropathy (defined as 25% change in serum creatinine) using sodium bicarbonate compared with sodium chloride was 11.9%, resulting in a number-needed-to-treat of 8.4 patients to prevent 1 case of renal failure. A recent meta-analysis and systemic review of the literature confirmed that sodium bicarbonate hydration decreased the risk of CIN, without a significant risk of renal replacement therapy, in hospital mortality or congestive heart failure compared to controls.28a Similar results were seen for risk of CIN when sodium bicarbonate was compared to normal saline alone (OR, 0.39; 95% CI, 0.20 to 0.77). However, when sodium bicarbonate/N-acetylcysteine combination was compared with N-acetylcysteine/normal saline combination, no improvement in outcome was determined (OR, 0.68; 95% CI, 0.34 to 1.37). Results have been conflicting for the use of N-acetylcysteine (NAC) in more than 40 trials and 13 meta-analyses; the most meticulous meta-analysis does not support the use of NAC to reduce the risk of CIN.28b,28c On the other hand, the use of NAC is not associated with any major adverse effects (except with high-dose intravenous use which carries a risk of anaphylactoid reactions28d); thus its use is generally not contraindicated. It is, however, not considered a substitute for hydration.
Patients with eGFR <60 mL/min receiving intra-arterial contrast should therefore receive IV hydration with sodium bicarbonate. The initial intravenous bolus is 3 mL/kg bolus (MAX 300 mL) 1 hour prior to procedure AND 1 mL/kg/hour (MAX 100/mL/hr) during and for 6 hours post-procedure.14 The use of oral NAC at a dose of 1200 mg twice daily on the day before and the day of contrast administration (total of 2 days) could be optional but not considered additive.13 Use of the lowest concentration of contrast (<5 mL/kg) to achieve satisfactory image quality is encouraged. Dilute contrast can often be used without affecting image quality. Reasonable attempts should be made to avoid repeat contrast injections within 72 hours. Diuretics should be routinely withheld on the day of contrast injection. Metformin is not a risk factor for CIN and the injection of contrast is not contraindicated in patients taking this medication. However, serious complications (lactic acidosis) may rarely occur in patients taking metformin who subsequently develop acute kidney insufficiency. For this reason, it is recommended that metformin be stopped 48 hours before any contrast injection.
Determination of Approach
Common iliac artery (CIA) disease is generally treated through an ipsilateral, retrograde approach. If the CIA is occluded, contralateral flush catheter placement should be considered so that a complete diagnostic study can be performed before any intervention; this technique also provides access to protect the contralateral CIA from injury during ipsilateral CIA intervention. A complete diagnostic arteriographic examination includes a study of the aorta to exclude abdominal aortic aneurysm, oblique pelvic imaging of the iliac bifurcations to determine internal iliac artery patency and origin, and adequate views to evaluate CFA bifurcation disease. In general, the contralateral oblique projection shows the iliac artery bifurcations, whereas the ipsilateral oblique projection best displays the profunda origins at the femoral artery bifurcations. Finally, complete imaging of the infrainguinal runoff is requisite before inflow intervention. If imaging of the runoff is done only after completion of the intervention, differentiating preexisting distal occlusive lesions from emboli may be problematic. External iliac disease is generally better approached from the contralateral side because it permits more extensive treatment of the external iliac artery (EIA) into the proximal portion of the CFA if needed (Fig. 112-4).
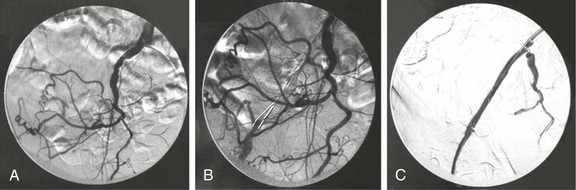
Figure 112-4 A, External iliac artery occlusion approached through the contralateral common femoral artery. B, Subintimal crossing of the complete occlusion with a reentry catheter. C, Contralateral placement of a bare metal stent into the external iliac artery, allowing for placement in the proximal portion of the common femoral artery.
The procedure begins with placement of an arterial sheath to facilitate catheter exchanges. The lesion is crossed with the use of a catheter–guide wire combination. An angle-tip catheter with a floppy-tipped guide wire is used to cross the lesion first, followed by the catheter. In difficult cases, hydrophilic guide wires may be used (see Fig. 112-4B). After advancing the catheter across the lesion, the wire is removed, and free aspiration of blood ensures that the catheter tip is intraluminal.
Determination of Hemodynamically Significant Lesion
Pressure measurements across the lesion should be obtained by connecting the hub of the catheter and/or the side arm of the vascular sheath to the intra-arterial pressure monitor. Alternative options include the “pullback method,” in which an end-hole catheter is withdrawn from proximal to distal across the lesion over a 0.014-inch wire. Most consider a peak-to-peak systolic pressure gradient of 10 mm Hg or greater at rest to be hemodynamically significant. In the absence of a resting gradient, intra-arterial nitroglycerin (100 to 200 µg) or papaverine (25 mg) can be administered distally to reveal the significance of a lesion. The maximal increase in pressure gradient occurs 20 to 40 seconds after vasodilator injection. If the systolic pressure gradient increases to above 10 mm Hg, the lesion may be considered for treatment.
Techniques to Recanalize Chronic Totally Occluded Iliac Arteries
Contralateral Approach
When attempting to recanalize an occluded common iliac artery in a retrograde fashion, the guide wire frequently follows a subintimal path. Once this has occurred, it may be difficult to redirect the guide wire into the lumen. An antegrade approach from the contralateral CFA is frequently successful, especially if there is a CIA stump and the CIA is not flush occluded. A hooked catheter is used to probe the occlusion. The lesion can then be crossed with the use of hydrophilic guide wire and a supporting catheter. As soon as the guide wire has crossed the obstructive lesion and lies within the ipsilateral EIA lumen, a catheter can be advanced over the wire and intraarterial placement can be confirmed. Depending on the proximity of the occlusion to the aortic bifurcation, a crossover sheath can be placed over a stiff guide wire or the wire can be snared from the ipsilateral CFA and a sheath can be placed in a retrograde fashion.
Brachial Approach
In the presence of a flush CIA occlusion, a contralateral femoral approach to cross the lesion is frequently unsuccessful; transbrachial or ipsilateral retrograde femoral approaches are more likely to achieve success. A brachial approach reduces the risk of creating or extending an aortic dissection and provides better “pushability.” The presence of significant subclavian artery occlusive disease obviously limits this approach.
Reentry Wires and Catheters
The development of reentry wires and catheters has greatly increased the technical success of crossing complete arterial occlusions. Crossing wires and catheters designed to cross chronic total occlusions (CTO) typically involves a wire that, based on a mechanical drive system, potentiates passage of the wire through the true lumen of the CTO either by a vibrating or rotating action. The majority of these devices have been tested in the femoral-popliteal vascular bed, and their utility in the iliac vessels is uncertain.
The reentry catheters facilitate crossing CTO by a subintimal approach. No data have suggested any advantage to crossing an iliac CTO either through the native lumen or subintimally. The Outback reentry catheter (LuMend Inc., Redwood City, Calif) is a single-lumen catheter designed to facilitate access and positioning of a guide wire within the peripheral vasculature from a remote vascular entry site using a retractable needle to pierce the intima and gain access to the true lumen (Fig. 112-5). The Pioneer catheter (Medtronic, Minneapolis, Minn) works by a similar mechanism but contains an intravascular ultrasound probe in the distal portion that assists in orienting the reentry needle toward the flow lumen. To use this catheter, a 300-cm–long, 0.014-inch guide wire is passed subintimally beyond the CTO. Typically, a .035-inch wire is used to make the initial subintimal plane and then exchanged for the .014-inch wire. The reentry catheter is then advanced beyond the CTO under continuous fluoroscopy. An angiogram is performed via the contralateral access to confirm traversal of the occlusion and positioning of the lateral exit port of the catheter at the aortic bifurcation proximal to the occlusion. In the case of the Pioneer catheter, this is done with IVUS. The precise location and orientation of the lateral exit port are confirmed by aligning the fluoroscopic guide. The nitinol cannula is then advanced forward through the lateral exit port under continuous fluoroscopy. Applying firm but guarded forward pressure while deploying the cannula may contribute to a successful puncture. Free passage of the 0.014-inch wire indicates true lumen access. This is confirmed by contrast injection through a catheter placed over the wire. The catheter can be exchanged for a 3-mm–diameter angioplasty balloon that is used to dilatate the subintimal tract and puncture site. This step enables a catheter to pass and allows exchange for a standard 0.035-inch guide wire to facilitate stenting. Stenting can then be performed in a conventional manner (Fig. 112-6).
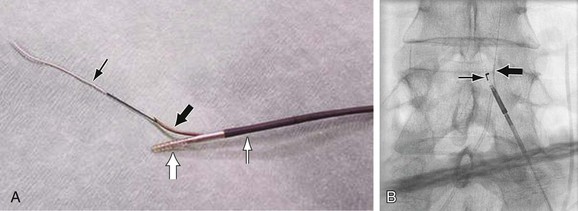
Figure 112-5 Reentry catheters. A, Outback LTD catheter. The cannula (large black arrow) is deployed, and the 0.014-inch guide wire (small black arrow) is advanced through it. The nosecone (large white arrow) has the radiopaque “LT” orientation marker. The catheter shaft is indicated by the small white arrow. B, Outback catheter. The cannula is deployed (large arrow), with free passage of the guide wire into the true lumen of the aorta. Note the “L” configuration (small arrow).
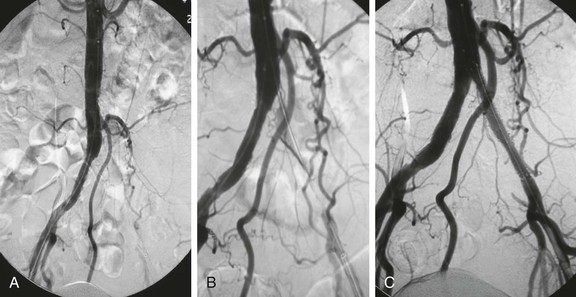
Figure 112-6 Results using a reentry catheter. A, Pelvic arteriogram showing complete occlusion of the left common and external iliac arteries. B, Glide catheter advancement into the aorta after reentry at the aortic bifurcation. C, Retrograde stent-graft placement into the common iliac artery. Note the contralateral bare metal common iliac stent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree