42 Aortic Vascular Interventions (Thoracic and Abdominal)
 Introduction
Introduction
Interventional Treatment of Aortic Dissection
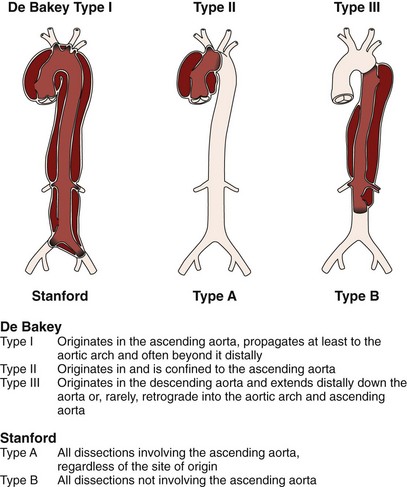
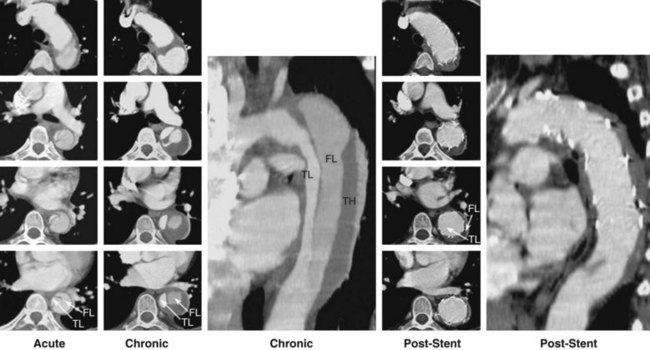
Acute aortic dissection is an uncommon but potentially catastrophic illness that occurs with an incidence of approximately 3.5/100,000 per year, with at least 8,000 cases per year in the United States.1 Around 0.5% of patients with chest or back pain suffer from aortic dissection.2 Within 14 days of onset, aortic dissection is considered acute, whereas it is chronic beyond a fortnight (Fig. 42-1). Men are twice as often affected as women by acute aortic dissection, with 60% classified as proximal (type A) and 40% as distal (type B) according to the Stanford classification.1 Historical data of untreated aortic dissection of the ascending aorta show a mortality rate of 1% to 2% per hour within the first 24 hours, resulting in a mortality rate of up to 50% to 74% in the acute phase.1 Acute type B dissection—when uncomplicated—is less frequently lethal, with survival rates in medically treated patients of 89% at 1 month, 84% at 1 year, and up to 80% within 5 years.1 Survival may be significantly improved by the timely institution of appropriate therapy; thus prompt clinical recognition and definitive diagnostic testing are therefore essential in the management of aortic dissection. Conventional treatment of Stanford type A (De Bakey types I and II) dissection (Fig. 42-1) consists of surgical reconstruction of the ascending aorta with complete or partial resection of the dissected aortic segment. Therefore, in type A dissections, interventional endovascular strategies have no clinical application except to relieve critical malperfusion before surgery of the ascending aorta. This is done by distal endovascular interventions in cases of thoracoabdominal extension of a proximal dissection (De Bakey type I) with distal ischemic complications. Conversely, stent graft placement aims at remodeling of the thoracic descending aorta, typically in Stanford type B dissection, by sealing one (or multiple) proximal entry tears with a Dacron-covered stent, thus initiating thrombosis of the false lumen.3–6 In addition, reconstruction of a collapsed true lumen might result in re-establishment of side branch flow (Fig. 42-2). Various scenarios of malperfusion syndrome are amenable to endovascular management. These include static or dynamic (by intima invagination) collapse of the aortic true lumen (so-called pseudocoarctation (Fig. 42-3), static or dynamic occlusion of one or more vital side branches, and enlarging false aneurysm due to patent proximal entry tear. Although peripheral pulse deficits can be acutely reversed with surgical repair of the dissected thoracic aorta in many cases, patients with signs of mesenteric or renal ischemia do not fare well. Mortality of patients with renal ischemia is 50% to 70%, and as high as 87% with mesenteric ischemia.7–9 Surgical mortality rates in patients with acute peripheral vascular ischemic complications are similar to those of patients with mesenteric ischemia, reaching an 89% in-hospital mortality rate.10–13 Operative mortality of surgical fenestration varies from 21% to 61%, which has encouraged percutaneous interventional management by endovascular balloon fenestration of a dissecting aortic membrane to treat mesenteric ischemia, a concept discussed as a niche indication in such complicated cases of malperfusion.12–14 Management of Stanford type B (De Bakey type III) dissection with the use of endovascular stent grafts is evolving slowly in anticipation of an unknown risk of paraplegia from spinal artery occlusion, as seen in up to 18% of cases after open surgery.13,14 With further technical improvement, a large series of patients has now been successfully treated in various specialized centers by endovascular stent graft placement covering entry tears in the descending aorta and even in the aortic arch. Recent studies have demonstrated that closure of proximal entry tears is essential to reconstruct the aortic wall and reduce total aortic diameter. Entry tear closure promotes depressurization of the false lumen, thrombus formation in the false lumen (Fig. 42-4), and remodeling of the entire aorta.4,5,14 In the near future, combined surgical and interventional procedures even for proximal dissection are likely to evolve.15–17
Current Indications for Endovascular Aortic Interventions
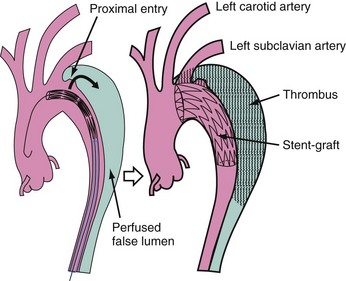
Significant perioperative morbidity and mortality in acute, complicated type B dissection has prompted alternative therapeutic concepts. Thoracic endovascular aortic repair (TEVAR), even though an off-label indication for dissection, has been approved by the U. S. Food and Drug Administration for aneurysmal disease of the aorta. The natural course of aortic dissection is determined by two elements: early complication and late events. In the acute phase, TEVAR has been shown to abrogate impending rupture and relieve static and dynamic malperfusion. Later benefit appears to be false lumen thrombosis, mitigating the risk of aneurysmal dilation and subsequent rupture. Thus, in 2010 the role of interventional management of static or dynamic obstruction of aortic branch arteries in complicated and complex distal dissection is settled. Static obstruction of a branch can be overcome by placing endovascular stents in the ostium of a compromised side branch, and dynamic obstruction may benefit from stents in the aortic true lumen, sometimes combined with side branch stenting and preferentially without any additional balloon fenestration, because fenestration does not improve stress and tension on the thin aortic wall.18 Sometimes bare stents deployed from the true lumen into side branches are useful to buttress the flap in a stable position.19 In rare cases, fenestration may be helpful to create a reentry tear for the dead-end false lumen back into the true lumen, with the aim of preventing thrombosis of the false lumen and compromise of branches fed exclusively from the false lumen; however, this concept lacks clinical proof of benefit. Conversely, fenestration increases the long-term risk of aortic rupture, because a large reentry tear promotes flow in the false lumen and provides the basis for its aneurysmal expansion. There is also a risk of peripheral embolism from a patent but partly thrombosed false lumen.19,20 The most effective method to avoid an enlarging false lumen is the sealing of proximal entry tears with a customized stent graft (Fig. 42-5); the absence of a distal reentry tear is desirable for optimal results but is not a prerequisite. Compression of the true aortic lumen cranial to the main abdominal branches with distal malperfusion (pseudocoarctation) is usually corrected by stent grafts that expand the compressed true lumen and improve distal aortic blood flow.4,5,12,14 Depressurization and shrinking of the false lumen is the most beneficial result to be gained, ideally followed by complete thrombosis of the false lumen and remodeling of the entire dissected aorta (see Fig. 42-2).16 Like previously accepted indications for open surgical repair in complicated type B dissection, scenarios such as intractable pain with descending dissection, rapidly expanding false lumen diameter, extra-aortic blood collection as a sign of imminent rupture, and distal malperfusion syndrome are at present accepted indications for emergent stent graft placement.16,17,19–21 Moreover, even late onset of complications such as malperfusion of vital aortic side branches is likely to justify endovascular stent grafting of an occlusive lamella to improve distal true lumen flow as a first option, with surgery employed only after an unsuccessful attempt, considering that surgical repair has failed to prove superior to interventional treatment even in uncomplicated cases. In complicated cases, the concept of endoluminal treatment is currently replacing open surgery in advanced aortic centers.3–5,19–22 Even in some cases of retrogradely extended type A dissections, TEVAR is feasible as a primary approach to seal the entry or as a secondary step after open repair of the ascending aorta.16 Open surgery may include an “elephant trunk” or transposition of arch vessels to allow for optimal landing zones for endovascular completion in a hybrid approach.23,24 A summary of potential treatment options is given in Table 42-1.
TABLE 42-1 Distribution of Differential Therapeutic Strategies in Aortic Dissection
Stable Acute Type B Aortic Dissection
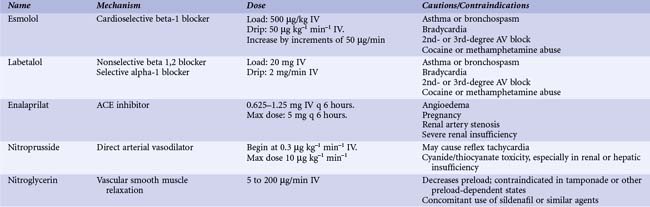
Patients with suspected acute aortic dissection should be admitted to intensive care and subjected to immediate diagnostic evaluation. Reduction of systolic blood pressure to 100 to 120 mm Hg with an eye on renal function and pain relief are initial priorities and are usually achieved by morphine sulfate and intravenous beta-blocking agents with or without vasodilating drugs such as sodium nitropusside at a dose of 0.3 µg/kg per min or angiotensin-converting enzyme inhibitors (Table 42-2). Additionally, heart rate should be kept low; a heart rate below 60 beats per minute significantly decreases secondary adverse events (aortic expansion, recurrent aortic dissection, aortic rupture and/or need for aortic surgery) in type B aortic dissection compared to a conventional rate of more than 60 beats per minute.25 Once both stable blood pressure and symptom relief are manifested, a patient with uncomplicated type B aortic dissection can be discharged (usually within 14 days) on oral drugs; clinical and imaging follow-up should be advised at 3 and 6 months and annually thereafter. In 384 patients with type B dissections from the International Registry of Aortic Dissection (IRAD), 73% were managed medically with an in-hospital mortality of 10%.1,26 Short-term survival rates were 91% at 1 month and 89% at 1 year. The reported long-term survival rate with medical therapy varies between 60% and 80% at 4 to 5 years and is around 40% to 45% at 10 years.1,26 Potential beneficial effects of early stenting are being studied in the ongoing ADSORB (Acute Uncomplicated Aortic Dissection Type B: Evaluation Stent graft Placement or best medical Treatment Alone) trial.27
Unstable Acute Type B Aortic Dissection
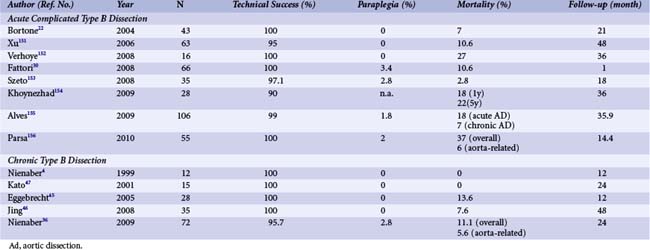
About 30% to 42% of acute type B aortic dissections are complicated, as evidenced by hemodynamic instability or peripheral vascular ischemia, and have an unpredictable outcome.26 Acute lower limb and visceral ischemia has been reported in 30% to 50%; malperfusion syndrome occurs frequently with extended dissections, with mortality between 50% and 85% if untreated.28 However, operative mortality in patients with acute aortic dissection complicated by renal ischemia has been reported around 50% and even 88% with mesenteric malperfusion.29 Conversely, of 571 patients with acute type B aortic dissection in IRAD, 390 were treated medically; among 125 complicated cases, 59 underwent standard open surgery and 66 were subjected to TEVAR. In this case, in-hospital mortality was significantly lower with TEVAR than after open surgery (10.2% vs. 33.9%; P = 0.002) (Fig. 42-6).30 The PETTICOAT (Provisional Extension to Induce Complete Attachment) concept takes the idea of endothoracic reconstruction even further by extending the stent graft scaffold distally with open-cell bare metal stents until distal malperfusion is corrected.31 With this concept, aortic fenestration maneuvers or branch vessel revascularization with side branch stents are usually not needed and almost obsolete. A metanalysis has summarized 942 patients from 29 studies showing an in-hospital mortality of 9% with reinterventions in 10.4%.32 Emergency surgical conversion or periprocedural stroke was rare (0.6% and 3.1%, respectively), while the survival rate was 88% at mean follow-up of 20 months. A second metanalysis comprised 1,304 patients subjected to TEVAR for complicated acute type B aortic dissection, with technical success in 99% and a 30-day mortality of 2.6%.33 At late follow-up, false lumen thrombosis was documented in 92.9% of patients and surgical conversion was required in 0.8%, with endovascular reintervention performed in 1.6%. Retrograde extension into the ascending aorta and neurological complications were reported in 0.4% and 0.6%, respectively.33 Further results are listed in Table 42-3. In patients deemed unsuitable candidates for conventional open surgical repair, 1- and 5-year survival rates were 74% and 31% with TEVAR compared with 93% and 78% (P < 0.001) survival in patients considered candidates for conventional open repair.34 Interestingly, a comparison between endovascular treatment of complicated type B aortic dissection and medical therapy of uncomplicated type B dissections in 56 patients with a follow-up of 18.1 ± 16.9 months reported similar outcomes in both groups with a better midterm fate of the descending thoracic aorta in the stent graft group, with no associated paraplegia; there were no differences in the 5-year survival rate (86.3% in both groups).35
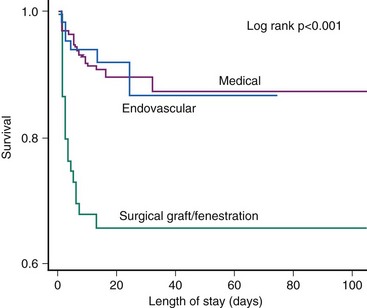
Figure 42-6 Comparison of medical, surgical, and endovascular treatment of complicated type B aortic dissection.30
Chronic Type B Aortic Dissection
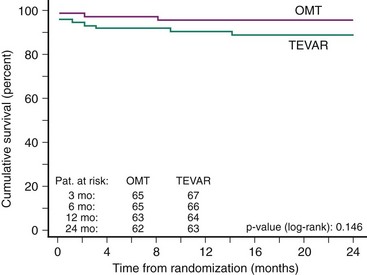
The evolution of an acute dissection to a chronic dissection involves progressive thickening of the intimal flap due to fibrosis. Additionally, more intimal tears are reported in chronic type B aortic dissection compared with acute dissection. The growth rate of the chronically dissected distal aorta is estimated to be between 0.10 and 0.74 cm per year depending on both initial aortic diameter and state of hypertension.1,24 Unfortunately the long-term outcome of medical therapy alone is suboptimal, with a reported 50% mortality at 5 years and delayed expansion of the false lumen in 20% to 50% of patients at 4 years.1,24 This expansion of the false lumen, for which an initial diameter beyond 4 cm and persistent perfusion of the false lumen were predictors, predisposes patients to aortic rupture or retrograde migration of the dissection toward the ascending aorta.1,24 Thus TEVAR should be considered when the aortic diameter exceeds 55 mm, there is persisting thoracic pain, or in the presence of uncontrolled hypertension and rapid growth of the dissecting aneurysm (>1 cm per year). Our group prospectively evaluated elective TEVAR in 12 patients with chronic type B dissection and compared the results with 12 matched surgical controls. Proximal entry closure and complete thrombosis of the false lumen at 3 months were achieved in all patients. Stent graft treatment resulted in no morbidity or mortality, whereas surgical treatment resulted in 4 deaths (33%; P = 0.04) and 5 adverse events (42%; P = 0.04)4; this has been confirmed by similar observations (Table 42-3). Whether prophylactic use of TEVAR in patients with chronic type B aortic dissections is superior to medical treatment alone was evaluated in the prospective randomized controlled INSTEAD (Investigation of STEnt grafts in Aortic Dissection) trial.36 A total of 140 patients in stable clinical condition at least 2 weeks after index dissection were randomly subjected to elective stent graft placement in addition to optimal medical therapy (n = 72) or to optimal medical therapy alone (n = 68). There was no difference in all-cause deaths and a 2-year cumulative survival rate of 95.5% ± 2.5% with optimal medical therapy versus 88.9% ± 3.7% with TEVAR (P = 0.15) (Fig. 42-7). Moreover, the aorta-related death rate was not different (2.9% vs. 5.6%; P = 0.68), and the risk for the combined endpoint of aorta-related death and progression was similar (P = 0.65). Aortic remodeling (with true lumen recovery and thoracic false lumen thrombosis) occurred in 91.3% of patients with TEVAR versus 19.4% of those who received medical treatment (P < 0.001), which suggests ongoing aortic remodeling. Initial (30-day) mortality is 10% or less with medical therapy in acute uncomplicated type B dissection,1,24 and data from the INSTEAD trial suggest no prognostic advantage of TEVAR within 2 years compared with monitored medical therapy for uncomplicated chronic type B dissection; these observations indicate that TEVAR should be reserved for complicated cases of acute or chronic descending thoracic aortic dissection or for those in which medical management has failed.
Technique of Aortic Stent Graft Placement
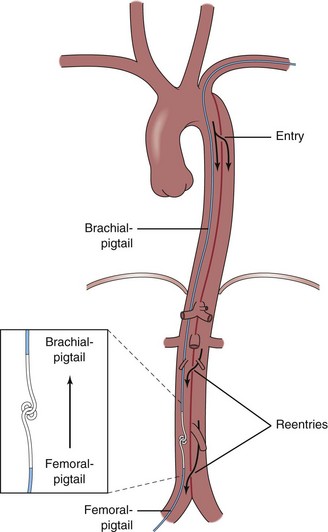
Aortic stent grafts are primarily used to reconstruct the compressed true lumen cranial to major aortic branches and to increase distal aortic flow. Therefore proximal communications should be sealed to direct flow into the true lumen, depressurize the false lumen, and induce thrombosis in the false lumen with fibrotic transformation and subsequent remodeling of the aortic wall. Stent graft placement across the origin of the celiac, superior mesenteric, and renal arteries may lead to fatal organ failure. Based on the measurements obtained during angiography, transesophageal echography (mandatory for the detection of small entries), contrast-enhanced spiral computed tomography angiography (CTA) (the best technique for unstable patients in an emergency situation), magnetic resonance angiography (MRA) (contraindicated for patients with pacemakers or implantable defibrillators), or intravascular ultrasound, customized stent grafts are available to both scaffold up to 20 cm of dissected aorta and cover major tears. The procedure is best performed in the catheterization and imaging laboratory using digital angiography and general anesthesia. The femoral artery is the most popular access site and can usually accommodate a 24-Fr stent graft system. In the Seldinger technique, a 260-cm stiff wire is placed over a pigtail catheter that is navigated with a soft wire in the true lumen under both fluoroscopic and transesophageal ultrasound guidance. In complex cases with multiple reentries in the abdominal aorta, the “embracement technique,” using two pigtail catheters, is useful (Fig. 42-8). A pigtail catheter that has been installed in the true aortic lumen via the left brachial artery picks up the femoral pigtail catheter in the true lumen of the abdominal aorta and pulls it up into the aortic arch. This procedure ensures definite positioning of the stiff guidewire in the true lumen, which is essential for correct deployment of the stent graft. The stent is carefully advanced over the stiff wire, and the launching of the stent graft is performed with systolic blood pressure lowered to 50 to 60 mm Hg by infusion of sodium nitroprusside or by rapid right ventricular pacing to prevent dislodgement.37,38 After deployment, short inflation of a latex balloon can improve apposition of the stent struts to the aortic wall, but only if proximal sealing of thoracic communications is incomplete. Paraplegia may occur after use of multiple stent grafts, but this still appears to be a rare phenomenon, especially when the stented segment does not exceed 16 cm. Both Doppler ultrasound and contrast fluoroscopy are instrumental for documenting the immediate result or initiating adjunctive maneuvers. For thoracic aortic aneurysms or ulcers, the navigation of wires and instruments is easier, but dual imaging using ultrasound and fluoroscopy simultaneously is equally important. A frequent anatomical consideration is the short distance between the origin of the left subclavian artery (LSA) and the primary tear in type B dissections. Coverage of the ostium to the LSA must sometimes be accepted in order to perform endovascular aortic repair in this aortic pathology adjacent to the LSA. According to observational evidence, prophylactic surgical maneuvers are not imperative or always required for safety reasons but may be relegated to an elective measure after an endovascular aortic intervention if intolerable signs or symptoms of ischemia occur.39 However, before intentional LSA occlusion, careful attention must be paid to potential supra-aortic variants (e.g., presence of a lusorian artery, a nonintact vertebrobasilar system, dominant left vertebral artery) that originate directly from the aortic arch and other pathologies recognized during preinterventional vascular staging.
Retrograde Type A Thoracic Aortic Dissection
TEVAR is associated with complications; new, formerly unexpected complications have emerged, such as endoleak, graft migration, device separation, and retrograde type A thoracic aortic dissection (rATAD). A European multicenter registry of 4,750 procedures estimated the incidence of rATAD at 1.33%, with 25% being asymptomatic cases40; one single center recently reported a 2.5% rate of rATAD (n = 11, among whom 3 patients had Marfan’s syndrome).41 Interestingly, rATAD developed intraoperatively in 2 patients, 2 hours after the procedure in one patient, at 1 week in 1 patient, and in 7 patients a month after TEVAR; of these cases, 8 were converted to open surgery while 2 received medical treatment.41 Open surgery is the treatment of choice in an effort to tackle such potentially fatal complications; however, the procedure-related mortality following rATAD surgery was itself between 20% to 57%.40,41 With the mechanisms of rATAD after TEVAR still unclear, observational evidence suggests that rATAD may be due to several causes (procedure-related, oversize ballooning, device-related, unfavorable aortic dissection anatomy, and natural progression of initial aortic dissection). As far as TEVAR-related factors are concerned, injury from proximal bare spring was suspected with outward pointing radial force. Lack of conformability of stent grafts when passively bent at the aortic arch may cause traumatic strain to the wall and create a tear. Balloon dilation after TEVAR can cause injury to the inner layers and retrograde extension. Indeed, additional balloon dilation was performed in 11 cases of rATAD (23%).40 Oversizing of the stent graft by more than 20% in relation to the landing zone diameter is also considered a risk factor for rATAD. Finally, genuine fragility of the aortic wall may predispose to rATAD as a sign of natural disease progression. Even under medical management, newly developed type A dissections were observed in 4 of 180 and in 5 of 66 patients under medical treatment for acute type B dissection.42,43
Timing of Endovascular Repair
The optimal timing for endovascular intervention in type B dissections remains controversial. Bortone et al. favor an early intervention within 2 weeks of the initial diagnosis; stent graft placement was successful in all patients referred for intervention within the first 2 weeks.22 A high rate of reverse remodeling is likely when the patient is treated early after development of the dissection flap. With the passage of time, the dissection flap becomes more fibrosed, thickened, and matured—that is, less compliant to TEVAR. Shimono et al. reported that complete obliteration and resolution of the false lumen following endovascular stent graft treatment was more frequently achieved in cases of acute aortic dissection compared with those of chronic aortic dissection (70% vs. 38.5%).44 Conversely, others have observed higher mortality rates in patients with acute type B aortic dissection.45,46 Morphological changes of the initially fragile dissecting membrane to a more fibrotic and seemingly stable membrane in the chronic phase are critical for endovascular repair, suggesting TEVAR to be safer after a minimum of 4 weeks following the onset of aortic dissection but before the chronic stage.47 Additionally, the more stable clinical status of patients in the chronic phase of aortic dissection may be an important determinant of better survival after TEVAR. Towing to the lack of prospective randomized data comparing immediate and delayed intervention in various clinical and anatomical constellations, no general recommendation has been issued with respect to the timing of endovascular treatment; observational evidence, however, may favor an early intervention in the window of aortic plasticity when justified by a low complication rate.
 Descending Thoracic Aortic Aneurysm
Descending Thoracic Aortic Aneurysm
Endovascular Repair by Stent Grafts
Thoracic aortic aneurysms, classified according to Crawford and Safi (Fig. 42-9), occur predominantly in the elderly and therefore have been increasing in incidence as the population ages and diagnostic capabilities advance.48 With an incidence of 6 to 10 per 100,000 person-years, thoracic aortic aneurysms (TAAs) are less common than abdominal aortic aneurysms (AAAs) but remain life-threatening.48–50 The distribution of aortic segments was as follows: the ascending aorta was involved in 51%, the aortic arch in 11%, and the descending thoracic aorta in 38%. One-quarter of the patients had concomitant infrarenal aneurysmal aortic disease and up to 13% had multiple aneurysms, whereas the risk of having a TAA when AAA was diagnosed was between 3.5% and 12%.50 The pathogenesis of aortic aneurysms has not been established to its full extent, but it is believed to be multifactorial and to include atherosclerosis, increased tissue protease activity, antiprotease deficiency, mechanical factors, inflammatory disorders, infection, and genetic collagen defects, such as Marfan’s syndrome and Ehlers-Danlos syndrome. Up to 20% of patients with an aneurysm have a first-degree relative with the same disorder.51 The natural history of TAAs is one of progressive expansion and weakening of the aortic wall, leading to eventual rupture.52–54 Initial aneurysmal size can also be an important predictor of aneurysm growth; a study based on 721 patients supported the fact that TAA size had a profound impact on risk for rupture, with an annual rate of 2% in aneurysms <5 cm, 3% in aneurysms 5 to 5.9 cm, and 7% for aneurysms beyond 6 cm. Therefore the risk appears to rise abruptly as thoracic aneurysms reach a size of 6 cm.55 Beyond this dimensional view, nondimensional variables with an impact on expansion rate and risk of rupture should also be evaluated. In a multivariate regression analysis, the Mount Sinai group identified older age, the presence of even uncharacteristic pain, and a history of chronic obstructive pulmonary disease (COPD) as independent risk factors for rupture of TAA.56 With an associated mortality rate of 94%, TAA rupture is usually a fatal event.49,57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree