Aortic Valve Repair
Martin Misfeld
Friedrich W. Mohr
Introduction
Aortic valve repair is an old technique, which has been performed since the beginning of open heart surgery, to treat different aortic valve pathologies. Early results were discouraging due to a lack of understanding of the underlying valve pathology and the lack of perioperative valve assessment by echocardiography. The introduction of the latter has made it possible to correctly assess the underlying aortic valve pathology, more accurately predict the surgical technique to be used, and precisely evaluate the result of surgical repair.
Aortic valve repair can be divided into the following groups: (1) Restoration of aortic root geometry with aortic valve sparing techniques (remodeling and reimplantation technique). (2) Repair of individual structures of the aortic root.
The combination and modification of both techniques is common and is usually individualized to the valve pathology.
This chapter will focus on reconstructive surgery of isolated aortic root structures. Aortic valve sparing techniques (remodeling and reimplantation technique) are addressed in Chapter 9.
Anatomy
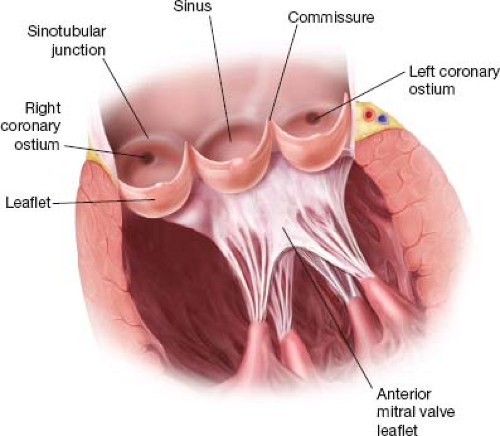
The aortic root has kindled great scientific interest ever since Leonardo da Vinci (1452–1519) made his drawings of the aortic root in the 15th century. The aortic root is composed of an annulus proximally and a sinotubular junction distally with three commissures, interleaflet triangles, sinuses of Valsalva, and cusps each between them. The cusps were originally named according to their anatomical position as posterior, right and left (British Terminology Anatomical System) or anterior and right and left posterior (International Terminology Nomenclatura Anatomica). In the 1950s, surgeons adopted a simpler terminology of noncoronary, right and left coronary leaflet based on their relation to the coronary ostia. Although the Nomina Anatomica (1980) describes the atrioventricular valves as having leaflets and the arterial valves as having semilunar valvules, most of the publications used the term “leaflets” for the structure suspended in the lumen between the commissures of the aortic root. Figure 3.1 depicts structures of the aortic root.
Terminology and Definition
Although aortic valve disease is the commonest valvular disorder, definitions of aortic root structures vary enormously. A clear definition and standardization, as to which segments of the ascending aorta form a part of the aortic root and which components of the aortic root form the aortic valve, is mandatory. This will ensure a uniform interpretation of outcome data with respect to clinical characteristics and surgical techniques, especially if the data is generated by nonmedical personnel. Therefore, an overall consensus regarding the nomenclature of aortic root structures, which is still lacking, is necessary.
The following definitions will be observed in this chapter:
Aortic root: Part of the ascending aorta, including the aortic valve and composed of the annulus, sinuses of Valsalva, sinotubular junction, intercusps, trigones, and cusps.
Aortic valve: Part of the aortic root, composed of three cusps.
Cusps: The mobile components of the aortic valve that separate the aorta from the ventricle.
Annulus: Also called the “functional” annulus. It is a virtual circular ring. Its height is defined by the nadirs of the semilunar cusp attachments. (The attachment of the cusps to the wall of the aorta: Semilunar cusp attachment.)
Sinotubular junction: The most distal part of the aortic root at the tip of the commissures.
Sinuses of Valsalva: Bulges of the aortic root, being bordered by the sinotubular junction and the semilunar cusp attachment.
Commissure: The distal parts of the cusps’ attachment to the aortic wall.
Triangle: The tissue between the cusp attachments and the annulus.
With regard to echocardiography:
Length of cusp coaptation: The maximal length of which two cusps run parallel. Target length after repair should be >8 mm.
Height of cusp coaptation: The maximal height of cusp coaptation measured from the level of the “functional” annulus to the free edge of the cusps. The coaptation of the cusps should be, therefore, longer than the length of cusp coaptation and should start above the level of the “functional” annulus.
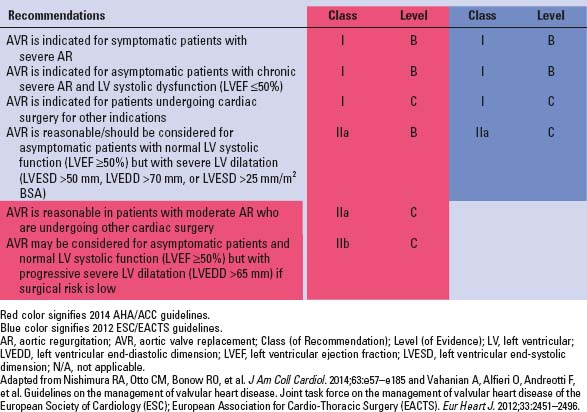
TABLE 3.1 Indications for Surgery in Patients With Severe Aortic Regurgitation | |
|---|---|
|
Indications for surgery in patients with aortic regurgitation depend on the following factors:
Severity of regurgitation
Clinical presentation
Indications for concomitant cardiac/aortic surgery
Deterioration of left ventricular function/enlarging dimensions
Indications for surgery as per the AHA/ACC and ESC/EACTS guidelines are mentioned in Table 3.1.
Besides patient factors, the indications for reconstructive aortic valve surgery depend a lot on the surgical experience of the team performing these procedures. In this context, it is of utmost importance that an accurate assessment of the quality of the tissue (cusps and aortic root) is made by an experienced surgeon, so as to achieve long-term durability after aortic valve repair. Therefore, any calcification, severe fibrosis, and/or aortic wall thinning should be carefully addressed and, if possible, corrected during the repair.
Contraindications for aortic valve repair are the same as for any patient undergoing cardiac surgery and depend upon individual judgment of the surgeon and the expertise of the center performing the surgery.
Role of Echocardiography
Intraoperative transesophageal echocardiography is mandatory during aortic valve repair surgery. It involves the preoperative assessment of the aortic valve and the evaluation of the repair before the patient leaves the operating room.
Preoperative assessment of the underlying mechanism of aortic valve regurgitation as well as the evaluation of tissue quality (i.e., calcification, fibrosis, aortic wall thickness) enables the surgeon to plan the operation.
The important echocardiographic parameters related exclusively to the aortic valve and root are as follows:
Calcification and/or fibrosis of the cusps and/or aortic root
Dimensions of the aortic root at annular, mid-sinus, and sinotubular level
Aortic root geometry
Cusp mobility
Identification and classification of bicuspid aortic valves (BAVs)
Severity of aortic regurgitation
The direction of the regurgitant jet as assessed by color Doppler (central, eccentric)
The final decision regarding the suitability of the aortic valve for repair can only be made on valve inspection, when the tissue quality and aortic root geometry can be visually evaluated.
Intraoperative echocardiography is extremely important to identify and quantify residual valvular regurgitation following aortic valve repair. A residual aortic regurgitation >grade I should not be accepted, as it is a predictor of recurrence of severe aortic valve regurgitation over time. The echocardiography should, therefore, focus on the grade and characteristics of residual regurgitation, cusp mobility, and, therefore, on the absence of relevant aortic valve stenosis, and the length, plane, and height of leaflet coaptation. The length of coaptation should be ≥8 mm, and the plane of coaptation should be above the annular plane, so that the height of coaptation is longer than the length of coaptation!
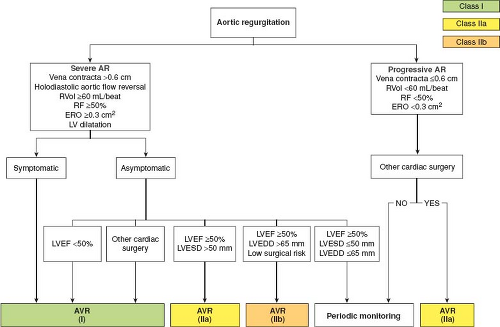
Echocardiographic data and indications for aortic valve reconstruction for chronic aortic regurgitation are depicted in Figure 3.2.
Medical Therapy
Patients with severe aortic valve regurgitation remain asymptomatic for a long time. Mild aortic regurgitation usually does not need any medical therapy. Although it does reduce afterload in patients with moderate or severe aortic regurgitation, the use of calcium channel blockers and angiotensin-converting enzyme inhibitors in asymptomatic patients with moderate or severe aortic valve regurgitation have not shown any beneficial effect in the absence of arterial hypertension. However, beta-blockers are recommended in patients with ascending aortic aneurysms to prevent/decelerate the progression of aortic dilatation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree