Chapter 11 SURGICAL INTERVENTION AND POSTOPERATIVE OUTCOME Timing of Surgical intervention Operative Mortality and Long-Term Survival Changes in Left Ventricular Hypertrophy, Geometry, and Function The primary determinant of disease severity in patients with valvular aortic stenosis (AS) is the degree of obstruction to left ventricular (LV) outflow. In addition, valve obstruction leads to secondary effects on the left ventricle, peripheral vasculature, and coronary artery blood flow that impact both the clinical presentation of disease and subsequent outcome. The pathophysiologic mechanisms involved in the initiation and progression of the disease process are described in Chapters 3 and 4. The rate of rise and fall of the antegrade velocity and the timing of the pressure gradient across the valve are also related to disease severity. With mild stenosis, the maximum velocity and maximum pressure difference across the valve occur in early systole, prior to the peak volume flow rate across the valve, at a time point corresponding to the maximum rate of flow acceleration.1,2 As stenosis becomes more severe, the maximum velocity and pressure difference occur later in systole, eventually coinciding with the maximum volume flow rate across the valve. In addition to stenosis severity, the shape of the velocity curve and timing of the pressure gradient may be affected by other factors that alter LV or aortic pressure, such as coexisting aortic regurgitation (AR) and increased systemic vascular resistance. The antegrade (or jet) velocity across the aortic valve is usually described in terms of the maximum instantaneous velocity. The maximum instantaneous velocity relates to the maximum instantaneous pressure gradient across the valve as stated in the Bernoulli equation. Although theoretically more complicated, the relationship can be simplified for most clinical applications so that the pressure gradient is equal to the velocity squared multiplied by 4 (i.e., “simplified Bernoulli equation”).3–6 At cardiac catheterization, the difference between peak LV and peak aortic pressures (the peak-to-peak gradient) is often reported. Because the peak LV and aortic pressures usually are not simultaneous, the difference between these two pressures is not a physiologic measurement and does not correspond to the maximum or any other instantaneous velocity obtained with Doppler echocardiography. Mean transaortic pressure gradients can be derived from Doppler echocardiographic data or invasive pressure recordings by averaging the instantaneous pressure gradients over the systolic ejection period. In adults with valvular AS, maximum and mean pressure gradients are linearly related, with a close correlation.7,8 The predictive value of mean gradient is also well established for both invasive and noninvasive assessment and this measurement should be included in diagnostic reports. The phenomenon of pressure recovery distal to the stenotic valve contributes to some of the confusion surrounding comparisons of invasive and noninvasive transaortic gradients, because Doppler velocities reflect the pressure drop in the orifice itself, whereas catheter pressure data may include pressure recovery distal to the orifice, depending on the exact location of the catheter relative to the stenotic orifice.9–11 The geometry of the flow obstruction in AS with its abrupt widening from the stenotic orifice to a comparatively large ascending aorta causes extensive turbulence with dissipation of kinetic energy into heat. This pattern precludes the occurrence of pressure recovery with clinically relevant magnitude in the majority of patients. Only in the case of a small aorta with a favorable ratio of orifice to cross-sectional aortic area—in this setting the occurrence of turbulences is reduced—will pressure recovery reach a magnitude that causes clinically relevant differences between Doppler gradient (pressure drop from ventricle to vena contracta) and net pressure gradient (drop from ventricular to distal, recovered pressure).9,11 In the clinical setting, this becomes likely when the aortic root diameter is smaller than 3 cm.11,12 In case of significant pressure recovery, the Doppler gradient overestimates the pathophysiologic consequence of a stenosis. In this case less proximal pressure and, consequently, workload are required to maintain an adequate peripheral pressure than in a clinical setting with the same Doppler gradient but no pressure recovery. Aortic valve area, defined as the extent of aortic valve opening in systole, provides a clinically useful measure of stenosis severity that is less dependent on volume flow rate than pressure gradients. Valve area can be calculated from Doppler data using the continuity equation based on the principle that the volume flow rates are equal just proximal to and in the stenotic orifice13–16 (see Chapter 6). Valve area also can be calculated at cardiac catheterization, using the Gorlin equation, on the basis of measurement of transaortic volume flow rate and mean systolic pressure gradient across the valve17–21 (see Chapter 7). Although less flow dependent than pressure gradients, aortic valve area also varies with transaortic volume flow rate, especially in patients with calcific stenosis.22–25 The unfused commissures allow variation in the degree of valve opening, depending on the interaction between the stiffness of the cusps and the force directed against the valve in systole.21,26–31 The variable opening of stiff aortic valve leaflets is not surprising, given the common echocardiographic observation in patients with dilated cardiomyopathy that changes in flow rate are associated with changes in the extent of aortic cusp opening, even in the absence of leaflet thickening. With aortic valve stenosis, the increase in LV outflow velocity with exercise may result in an increase in the extent of valve opening if the leaflets still have some flexibility. Initial concerns, that the observed increase was related to the mathematical assumptions of the calculations or to changes in the fluid dynamics across the valve, have been resolved by direct observation of valve opening, 32 so that most investigators now concur that leaflet opening varies with flow rate. With disease progression, the gradual increase in the degree of leaflet thickening and calcification eventually reaches a point at which valve area is fixed over the physiologic range of force that can be generated by the left ventricle. The time course of valve opening, or the rate of change in valve area during a single cardiac cycle, reflects valve stiffness, inertia, and elasticity. 33 Stenotic aortic valves open and close more slowly than normal valves 34 and the rate of change in valve area during systole is a predictor of clinical outcome. 35 Flow dependence of valve area becomes particularly important in the presence of low cardiac output most frequently due to LV dysfunction. Reduced opening forces may then cause a mildly or moderately stenotic valve to open to a valve area of only less than 1 cm2. The term “pseudosevere stenosis” has been proposed for this condition.36,37 Transvalvular gradient is typically low (mean gradient < 30-40 mm Hg) in this situation (low-flow, low-gradient AS). Although many patients with reduced LV function in a late stage of severe AS (afterload mismatch, see section titled Left Ventricular Pressure Overload) maintain a surprisingly high gradient (>40 mm Hg mean gradient), 38 some of them may also present with low gradients just because of severe flow reduction. Low-dose dobutamine can be used during echocardiography to stimulate myocardial contraction and increase flow rate. With increasing flow, one would assume that valve area increases with little change in gradient in the presence of pseudosevere stenosis and that primarily velocity and gradient should increase with less change in valve area when there is fixed severe stenosis. 37 The test, of course, requires that there is indeed contractile reserve. In addition to flow-related changes in geometric orifice area, it has been suggested that effective orifice area may increase with flow even without changes in anatomic area. 39 At normal flow rates, the kinetic energy of the fluid crossing the obstruction is sufficient to break down the vortex structures generated downstream from the stenosis and thus enables the formation of a large and well-established flow jet. However, at low flow rates, the reduction in kinetic energy may predispose to the formation of vortices, which tend then to squeeze the flow jet and thus the vena contracta, resulting in a smaller effective orifice. 39 The phenomenon is certainly less important in the presence of very small orifices but may become clinically relevant in moderately severe “low-flow AS.” These findings once more emphasize that clinical judgment should not rely only on the absolute number of calculated valve area but should take into account all available hemodynamic and morphologic information. 40 Some investigators have explored the concept of valve resistance as a measure of AS severity.31,41,42 Because valve resistance is calculated as the simple ratio of pressure gradient to flow across the valve, the underlying assumption of this approach is that there is a linear relationship between pressure gradient and transvalvular flow rate. This assumption is inconsistent with the Bernoulli equation, which assumes a quadratic relationship between pressure gradient and flow rate. Some disagreement persists as to the exact relationship between pressure and flow across a stenotic valve, but in fact, careful fluid dynamic studies support the concept of a quadratic relationship. 43 Although experimental studies suggest that valve resistance provides better discrimination of the extent of valve stiffness for valves 100 to 200 times stiffer than normal, disease progression and symptoms occur in the clinical setting in the range of valve stiffness from 20 to 100 times normal, a range where valve area provides better quantitation of disease severity. 44 Valve resistance has also been proposed for the evaluation of low-flow, low-gradient AS, with the argument that it is less flow dependent. 41 However, this concept cannot be supported by fluid dynamics theory, nor can it be supported experimentally or by clinical observation.11,29,43,45 The studies examining the issue demonstrated that valve resistance certainly has no advantages over valve area with regard to flow dependence. The fact that valve resistance may indeed obscure actual changes in valve area, because the resistance may change only slightly when valve area increases with increasing flow rate, makes it even less useful for the evaluation of AS. As a matter of fact, an increase in flow must necessarily result in an increase in resistance unless it is compensated by a concomitant increase in effective valve area.11,45 In addition, the calculation of valve “resistance” has no clear advantages over jet velocities, pressure gradients, and valve areas in predicting clinical outcome.46,47 The fluid dynamics of AS are more complex than those of mitral stenosis, in that the pressure gradient and volume flow rate across the valve depend on the force of LV contraction as well as the characteristics of the valve itself. Thus, another approach to describing AS severity is to estimate the total work performed by the ventricle in opening the aortic valve. In concept, total LV stroke work is calculated as the integral of flow times pressure. Effective stroke work (aortic pressure times flow) then is subtracted to yield the stroke work “lost” across the valve.48,49 Although LV stroke work loss does correlate with other measures of stenosis severity, it also varies with flow rate (even when normalized for stroke volume), is an unfamiliar concept for most physicians, and offers no obvious clinical advantages. In addition, the calculation of stroke work loss accounts mainly for the potential energy components of total work, whereas kinetic energy losses, which are more difficult to estimate, may be even more important in valvular AS. 29 Because aortic valve hemodynamics depend on aortic valve anatomy, LV mechanics, and the characteristics of the vascular system downstream from the valve, a complete description of AS severity would include all three of these components. Obviously, this type of descriptor is conceptually complex and may be difficult to derive in the clinical setting. A step toward an integrated descriptor of AS severity is the concept of ventricular-vascular coupling with the inclusion of components to describe the effect of the abnormal valve in the system. 50 Results of preliminary studies in this area are of interest but are not yet clinically applicable. The basic response of the left ventricle to the chronic and gradually progressive pressure overload of valvular AS is concentric hypertrophy. However, not all patients demonstrate hypertrophy, even with severe stenosis, and there are significant gender differences in the degree and pattern of hypertrophy.51–54 In pathophysiologic terms, LV hypertrophy occurs as a mechanism to maintain normal wall stress as LV pressure rises through an increase in wall thickness (see Chapter 5). Typically, contractility is normal and ejection fraction (EF) is preserved until late in the disease course. However, even when contractility is normal, LV systolic performance may appear to be impaired in patients with severe outflow obstruction for at least three reasons. First, EF may decline owing to the excessive increase in afterload, often termed “afterload mismatch.” Second, ventricular preload may be shifted to the left on the Starling curve because of a small, hypertrophied, noncompliant ventricle. Third, the temporal sequence of myocardial contraction is often asynchronous in pressure overload hypertrophy, with an “uncoordinated” ventricular contraction. The resultant fall in the peak rate of circumferential shortening correlates with an increase in systolic wall stress. 55 This pattern of discordant contraction, and the apparent decrease in ventricular function, resolves after relief of AS. When LV mass measurements are normalized for body size and gender, hypertrophy is seen in 54% of men and 81% of women with AS. 52 The pattern of hypertrophy in women with AS is characterized by a small ventricular chamber with increased wall thickness, normal or hypercontractile systolic function, and early diastolic dysfunction.51–54,56 In men with AS, the more common pattern is a normal or only mildly increased wall thickness and impaired systolic function. Diastolic dysfunction occurs early in the disease course of AS 57 in association with an increase in the total collagen volume of the myocardium and an increase in the orthogonal collagen fiber network. 58 As for ventricular hypertrophy, significant gender differences in diastolic function are seen. Specifically, men have a higher constant of myocardial stiffness in association with a greater degree of endocardial fibrosis and an abnormal myocardial collagen pattern. 56 Age also affects the severity of diastolic dysfunction, with more severe LV hypertrophy and diastolic dysfunction seen in elderly patients (>65 years). 59 In patients with AS, the need to correct for peripheral amplification if femoral artery, rather than central aortic, pressures are used for invasive calculation of valve area has long been appreciated. 60 However, there is little data on the influence of systemic factors on valve or ventricular function in these patients. Although LV afterload is predominantly affected by the severity of obstruction at the valvular level, both factors internal to the left ventricle and characteristics of the systemic vascular circuit also contribute to total afterload. 50 To date, few studies have evaluated the impact of systemic vascular resistance (or impedance), wave reflections, or aortic elastance on the hemodynamics of valvular AS. Prospective follow-up shows that pulmonary hypertension is common in patients with isolated AS. 47 In a series of 388 symptomatic patients with isolated AS, mild pulmonary hypertension was present in 35%, moderate in 50%, and severe (systolic pressure >50 mm Hg) in 15%. 61 In addition, a higher prevalence of moderate to severe pulmonary hypertension (seen in as high as 71% of patients) has been noted in some surgical series. 62 The degree of pulmonary hypertension correlates with LV end-diastolic pressure but not with the severity of AS or the LV EF. 61 The presence of pulmonary hypertension is a risk factor for cardiac surgery, 63 but pulmonary pressure usually returns to normal after valve replacement for AS, even when it was severely elevated.62,64 Abnormalities in coronary blood flow, even in the absence of significant coronary atherosclerosis, contribute to the clinical presentation and long-term outcome of patients with valvular AS. Although coronary artery size, and thus blood flow, is increased in patients with AS, the increase in coronary artery size often is inadequate for the increase in muscle mass and, in addition, coronary flow reserve is limited.65–68 Coronary flow reserve is most impaired in the subendocardium, with the severity of impairment related to AS severity. 69 After aortic valve replacement, coronary flow reserve improves in conjunction with regression of LV hypertrophy. 70 LV hypertrophy also is associated with decreased capillary density and increased diffusion distances. 71 Other factors that may impact coronary blood flow in patients with AS are a decreased diastolic perfusion time, impaired early diastolic relaxation, and increased diastolic wall stresses, all leading to a reduction in subendocardial blood flow. 72 Transthoracic echocardiographic evaluation of phasic coronary blood flow in adults with AS shows reversal of early systolic flow and delayed forward flow in diastole, both of which resolve after aortic valve replacement. 73 These findings were further elucidated in both transthoracic and transesophageal echocardiographic studies showing that systolic coronary flow decreases in inverse relationship to the increase in LV wall stress, whereas diastolic flow increases in direct relationship to the transaortic pressure gradient, with these changes being particularly marked in symptomatic patients.74,75 Coronary flow, measured by an intracoronary Doppler flow catheter, also shows retrograde systolic flow at rest, which correlates with the peak transaortic pressure gradient. 76 With stress induced by pacing and/or dobutamine, retrograde systolic flow increases, total systolic flow decreases, and forward diastolic flow increases in patients with AS; in normal control subjects, both systolic and diastolic flow increase proportionately. 76 These data suggest that an inadequate increase in total coronary blood flow in response to stress may contribute to the clinical presentation of AS, specifically the symptom of angina in patients with normal coronary arteries. Further imbalance in myocardial oxygen demand and supply occurs late in the disease course as LV wall stress (and oxygen demands) increase out of proportion to the increase in coronary blood flow. 77 Angina, in the absence of coexisting coronary artery disease (CAD), is associated with an increased LV wall stress as a result of inadequate hypertrophy in conjunction with increased ventricular systolic pressures. 71 This increase in wall stress leads to an increase in myocardial oxygen consumption. The combination of increased myocardial oxygen demand and limited coronary blood flow leads to myocardial ischemia and symptoms of coronary regurgitation. Even asymptomatic patients with AS have a slight decrease in exercise tolerance in comparison with normative age standards. The hemodynamic response to exercise is characterized by a normal increase in heart rate to age-predicted maximums, but only a 50% increase in cardiac output. The increase in cardiac output is mediated by an increase in heart rate as stroke volume is unchanged or decreases slightly with upright exercise.23,24,29,30,48,78 Although total stoke volume does not increase, there is a rise in the maximum instantaneous and mean systolic flow rates across the aortic valve because the systolic ejection period shortens as a result of the increase in heart rate. Transaortic velocity, maximum gradient, and mean gradient increase as the flow rate rises, although the extent of increase often is less than predicted by the resting valve area.23,24,30,31,79 Measures of LV diastolic function are also abnormal with exercise in adults with AS. Micromanometer pressure recordings demonstrate that resting diastolic pressures are higher, diastolic pressures increase further with exercise, and both the rate of diastolic pressure decay and the isovolumic contraction interval fail to decrease with exercise, in comparison with observations in normal control subjects. 80 With exercise, valve area enlarges, on average, by 0.2 cm2, accounting for the smaller increase in jet velocity and gradient than expected for resting valve area.23,24,30 The increase in valve area with exercise allows ejection of a relatively normal stroke volume across the valve and an appropriate increase in cardiac output. As the disease becomes more severe and the leaflets become more rigid and stiff, the degree of valve opening is progressively limited, resulting in a drop in transaortic stroke volume and a failure of cardiac output to increase adequately with exercise.24,29 The increase in valve stiffness at adequate cardiac output results in a higher increase in gradient with exercise, which has been shown to be a predictor of outcome. 81 Valvular AS is a gradually progressive disease in which patients remain asymptomatic for many years.82–86 Typically AS is first diagnosed from the finding of a systolic murmur on auscultation. Because the increase in hemodynamic severity occurs slowly, many patients do not recognize early symptoms, emphasizing the importance of patient education in medical management, including a discussion of the classic symptoms of AS (e.g., heart failure, angina, and syncope). In addition, the clinician must carefully question the patient to elicit symptoms, specifically asking the patient to compare current activity levels with activities at a set point in the past. In particular, older patients often unconsciously tend to avoid activities that may cause symptoms and then still describe themselves as asymptomatic. The most common initial symptom of valvular AS is decreased exercise tolerance due to exertional dyspnea or fatigue.47,87 The mechanism of this symptom most often is an elevated LV end-diastolic pressure due to a noncompliant, hypertrophied ventricle. 88 Exercise intolerance may also be due to LV systolic dysfunction or coexisting CAD in some patients. Over time, exertional dyspnea may progress to frank heart failure, with symptoms seen at rest in patients with long-standing severe valvular obstruction. Some patients present with the sudden onset of heart failure or pulmonary edema, often in relation to an acute infectious process, anemia, or other hemodynamic stress or to new-onset atrial fibrillation. Exertional angina also is a common initial symptom in adults with valvular AS; it is due to an increase in oxygen demand by the hypertrophied myocardium, even in the absence of coexisting epicardial CAD.47,87 As mentioned, angina may be precipitated by other hemodynamic stresses, such as pregnancy, anemia, and febrile disease. The third classic symptom of AS is exertional lightheadedness or syncope. Several potential mechanisms of syncope in AS have been proposed, including ventricular arrhythmias and LV systolic dysfunction, but there is most support for an acute drop in blood pressure due to an inappropriate LV baroreceptor response.89–91 The elevated ventricular pressure activates baroreceptors, which mediate peripheral vasodilation. In the setting of a restricted aortic orifice, cardiac output fails to rise, so blood pressure falls and the patient losses consciousness. The key features in the physical examination of patients with suspected AS are palpation of the carotid pulse contour and amplitude; auscultation of the location, loudness, timing, and radiation of the systolic murmur; assessment of the splitting of the second heart sound; and examination for signs of heart failure.92,93 The timing and amplitude of the carotid pulse contour reflect central aortic pressure. As AS becomes more severe, the peak aortic pressure occurs later in systole (pulsus tardus) and the pulse amplitude is decreased (pulsus parvus). Both the timing and amplitude of the carotid pulse correlate with AS severity.94,95 However, the pulse contour is affected by factors other than stenosis severity, particularly in adult patients. 96 The pulse amplitude may be diminished with a reduced cardiac output and only mild to moderate stenosis, as a result of the low volume flow rate into the aorta, although the timing of the impulse is typically normal in this situation. Conversely, the pulse amplitude and timing may appear to be normal with coexisting atherosclerosis because the stiff vessels lead to a rapid and excessive rise in aortic pressure even when severe stenosis is present. Thus, a slow-rising, low-amplitude carotid pulse has a relatively high specificity for the diagnosis of severe valvular obstruction. However, the sensitivity of this finding is poor, and severe stenosis cannot be excluded in adults with an apparently normal carotid upstroke. The systolic murmur of AS is most often loudest at the base, over the right second intercostal space. In general, the loudness of the murmur correlates with jet velocity or pressure gradient.95,97,98 The presence of systolic thrill in the aortic region (i.e., a grade IV murmur) is highly specific for severe valvular obstruction. Conversely, severe stenosis is unlikely with a grade I murmur. Unfortunately, there is considerable overlap in disease severity with the intermediate grades of murmur (II-III), so further evaluation is needed, depending on the clinical setting.87,95 Besides the systolic pressure gradient across the valve, the loudness of the murmur is modulated by the volume flow rate across the valve, transmission of the murmur to the chest wall, and the direction of the turbulent jet. Thus, even with severe stenosis, the murmur may be soft if cardiac output is low or if obesity or lung disease diminishes its transmission to the chest wall. The murmur of AS radiates to the carotid arteries in the majority of patients as the turbulent jet is directed superiorly into the ascending aorta, allowing transmission of sound through the aorta to the carotids. In a minority of patients, the murmur radiates to the apex, a pattern referred to as the Gallavardin phenomenon. 99 An S4 gallop may be appreciated in many patients with AS, reflecting a greater atrial contribution to ventricular filling. 100 Other physical examination findings in AS patients depend on whether hemodynamic decompensation has occurred, leading to typical signs of heart failure. The chest radiograph may be entirely normal in patients with valvular AS, although dilation of the ascending aorta may be appreciated in some cases, even early in the disease course. Such aortic dilation has previously been called “poststenotic.” However, it is not related to hemodynamic severity and appears to be caused by intrinsic abnormalities of the aortic wall rather than by the stenosis itself, particularly in patients with bicuspid valves. 101 The cardiac silhouette typically is normal because LV hypertrophy due to an increased wall thickness with a normal chamber dimension is not evident on a standard chest film. Calcification of the aortic valve rarely is evident on chest radiography but may be seen with fluoroscopy in a high percentage of patients with severe valvular obstruction. 102 Mitral annular calcification, which often accompanies degenerative aortic valve disease, may also be seen. With long-standing disease, LV dilation and signs of heart failure are present. Radiographic evidence of pulmonary hypertension may also be evident late in the disease course. The classic electrocardiographic finding in AS is LV hypertrophy. However, many adults and children with severe AS do not have electrocardiographic criteria for LV hypertrophy.103,104 Other nonspecific electrocardiographic changes in adults with AS are left atrial enlargement, left axis deviation, and left bundle branch block. Although early studies suggested that T wave changes correlate with the degree of AS, this finding has not been reliable in clinical practice.105,106 Electrocardiographic changes with exercise, specifically ST depression, are common in adults with AS. Significant (>1 mm) flat or downsloping ST depression is observed in about two thirds of patients, even with only mild to moderate valve obstruction. Even when their resting electrocardiography findings are normal, half of patients with AS have ST depression with exercise. The presence or severity of ST changes with exercise in adults with AS does not correlate with the presence or absence of epicardial CAD. 47 The standard echocardiographic evaluation of a patient with known or suspected AS includes assessment of stenosis severity, degree of coexisting AR and LV size and function, estimation of pulmonary pressures, and identification of any other cardiac abnormalities. 47 With an experienced examiner, diagnostic data are obtained on transthoracic examination in nearly all patients. The most clinically useful measures of stenosis severity are maximum aortic jet velocity, mean pressure gradient (highly flow dependent), and continuity equation valve area (less flow dependent) ( Table 11-1). For details see also Chapter 6. Despite several potential limitations, the validity and accuracy of Doppler measures of AS severity for clinical purposes are well established both in comparison with catheterization data5,6,107–111 and in terms of clinical outcome.8,47 Nevertheless, it has to be emphasized that in clinical practice, careful consideration of all three measurements in conjunction with other findings, such as valve morphology and LV function, should be the basis for the final judgment of stenosis severity that will guide clinical management. Current guidelines define severe AS as a peak velocity higher than 4 m/s, a mean gradient greater than 40 mm Hg, and AVA less than 1.0 cm2,112,113 but it has to be noted that discrepant findings regarding these criteria are common in patients with the disease ( Table 11-2). Most frequently, a patient has a small AVA (<1.0 cm2) but nevertheless lower values for peak velocity (<4.0 m/s) and gradient (<30-40 mm Hg). Low-flow, low-gradient AS in the presence of LV dysfunction as one reason for this finding has already been discussed. The most challenging group, however, remains patients with small valve area and low gradients in the presence of preserved EF. TABLE 11-2 Classification of Aortic Stenosis Severity *In the presence of reduced stroke volume: An indexed stroke volume (SVi) ≤ 35 ml/m2 has been proposed as a cutoff value. The entity of severe paradoxical low-flow, low-gradient AS with preserved EF has been described, 114 with a stroke volume index of 35 mL/m2 or less being defined as a “low flow.” This situation may be observed in the presence of severe LV hypertrophy with a small LV cavity. Prior to diagnosis of severe paradoxical low-flow AS, it is essential to rule out potential underestimation of flow and, consequently, of valve area by the continuity equation (see previous discussion). Furthermore, it must be noted that currently used cutoff values for valve area and gradient are not entirely consistent. A mean gradient of 40 mm Hg requires, at normal flow rates, a valve area closer to 0.8 cm2 than 1.0 cm2. Finally, the patient with small stature may also have a small valve area but low gradient without having severe AS. In the initial retrospective study of this entity, a worse outcome was described in patients with paradoxical low-flow AS who were managed conservatively than in those undergoing aortic valve replacement. 114 In contrast, an analysis of data from the prospective Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) cohort described an outcome that was comparable to that for patients with moderate AS. 115 The populations of these studies differed markedly, however, perhaps explaining the differences in the results. Although it is premature to draw final conclusions with regard to the management of patients with low-flow, low-gradient AS with preserved EF, decisions need to be individualized and explanations other than the presence of severe AS must be carefully excluded when a small valve area and low gradients are found despite normal LV EF. 116 Coexisting AR is present in 70% to 80% of adults with predominant AS.7,47 LV chamber dimensions and volumes, wall thickness, mass, EF, and diastolic dysfunction are calculated by means of standard techniques. LV meridional and circumferential wall stress can be calculated from echocardiographic data in conjunction with a cuff blood pressure measurement, as described in Chapter 6. Although useful in clinical research studies, wall stress calculations are rarely performed routinely because these measurements are tedious to perform, and their clinical utility has not yet been convincingly demonstrated. Other important information derived from the echocardiographic examination includes left atrial size, pulmonary artery systolic pressure, right ventricular size and systolic function, and mitral valve anatomy and function. Mitral annular calcification is seen about 50% of adults with AS, 47 and about 90% of patients have mild coexisting mitral regurgitation, with a smaller number having moderate mitral regurgitation. In patients with rheumatic disease, evaluation of the severity of mitral stenosis and/or regurgitation is also needed for clinical decision making. Exercise testing can be safely performed in patients with minimal or no symptoms.29,47,117 The study should be promptly stopped if there is any decline in blood pressure, symptom onset, or the occurrence of significant arrhythmias. Exercise testing may be used to clarify symptom status in patients with equivocal symptoms, denial of apparent symptoms, or a decrease in exercise tolerance as perceived by other family members. A normal exercise test result indicates a very low likelihood of symptom development or other complications within the following 6 to 12 months.118–120 Symptoms on exercise indicate a high likelihood of symptom development or complications within 12 months in physically active patients, particularly those younger than 70 years. 120 Shortness of breath during exercise in patients with little physical activity in daily life, however, particularly the elderly, may be a nonspecific finding. Abnormal blood pressure response and/or ST segment depression has a low positive predictive value. 120 Stress testing is also indicated to objectively measure the exercise capacity and to define the parameters of a safe exercise program in the asymptomatic patient. Although patients with AS should not participate in competitive sports or extremely vigorous activities, they usually tolerate moderate levels of recreational activity well.121,122 Exercise electrocardiography is not helpful for the detection of coexisting CAD in patients with valvular AS. Although the increase in mean pressure gradient as assessed by exercise echocardiography has been reported to predict outcome and provide information beyond a regular exercise test,81,123 more data are required to validate this finding and support its use in clinical practice. Echocardiographic evaluation of the change in valve area with changes in flow rate in response to intravenous infusion of dobutamine may be helpful in the subgroup of patients with AS and significant LV systolic dysfunction who present with low gradient but small valve area.37,124–130 For a description of the hemodynamic principles, see the earlier discussion Valvular Hemodynamics. More data are required to confirm that the distinction between truly severe and pseudosevere AS by dobutamine stress echocardiography is clinically useful and should guide clinical management, but contractile reserve—defined as a stroke volume increase of 20% or more—has been shown to be a potent predictor of outcome. 126 However, contractile reserve, surprisingly, has not been found to be an independent predictor of postoperative LV function. 131 In several studies, patients without contractile reserve prior to surgery had a higher perioperative mortality, but those who survived aortic valve replacement were found to have an increase in EF similar to that in those with contractile reserve 131 and to have a significantly better 5-year survival than patients managed conservatively. 132 Measuring the degree of aortic valve calcification by multislice computed tomography (CT) may be useful for the evaluation of AS severity, especially in difficult cases, such as patients with reduced EF and those without contractile reserve. 133 Aortic stenosis severity is routinely quantified using Doppler echocardiography. Invasive measurement of the transaortic gradient and calculation of valve area using the Gorlin formula is needed only in cases in which echocardiographic data are nondiagnostic or inconclusive (see Chapter 7). In adults with valvular AS, obstruction to LV outflow develops gradually over many years.83,85,86 In many patients, AS is coincidentally diagnosed when echocardiography is performed for other reasons or after the finding of a systolic murmur on examination, while they are still asymptomatic. Asymptomatic patients are found across the whole spectrum of AS severity, including a significant number with severe AS. In some patients a substantial decrease in valve area and an increase in transaortic velocity occur prior to symptom onset. The occurrence of symptoms clearly presents a turning point in the natural history of the disease ( Table 11-3). Patients with congenital AS may become symptomatic in early childhood or adolescence; in particular, patients with unicuspid valves tend to present with early symptoms. Later, at young adult age—typically between 20 and 30 years—these patients may also present with symptoms due to restenosis after a surgical valvotomy in childhood.134,135 In the patient with congenital bicuspid stenotic aortic valve, surgery is typically performed between ages 50 and 70 years.136–138 In the adult patient with degenerative calcific valve disease, symptom onset may already have occurred at age 50 but typically occurs in the elderly, between 70 and 90 years. 138 Rheumatic AS becomes symptomatic over a wider age range, with patients most often presenting between age 20 and 50 years. In the absence of overt symptoms, clinical outcome with AS is excellent ( Figure 11-1). However, some investigators suggest that irreversible changes of the ventricular myocardium occur even prior to symptom onset. 139 FIGURE 11-1 Event-free survival with aortic valve disease. With conservative follow-up of asymptomatic patients with AS, the risk of sudden death is one of the major concerns. In three studies that included significant numbers of patients with nonsevere stenosis, no sudden death was reported. Otto et al 47 followed up 123 patients with an average peak velocity of 3.6 ± 0.6 m/s for 30 months. The two other series, with 51 140 and 37 patients, 141 had follow-up periods of 1.5 and 2.0 years, respectively. Only two studies reported the outcome of larger cohorts of patients with exclusively severe stenosis, as defined by a peak aortic jet velocity 4.0 m/s or higher. Pellikka et al 142 observed 2 sudden deaths among 113 patients during a mean follow-up of 20 months. Both patients, however, had experienced symptoms at least 3 months before death. In a later published study, which is the largest to date, 11 sudden deaths were observed among 622 patients who had been followed up for a mean of 5.4 years. 143 However, as the investigators state, medical follow-up was limited in about half of the patients. It thus remained unclear in this study whether these patients had eventually experienced symptoms in the months prior to death. Rosenhek et al 144 reported 1 sudden death that was not preceded by any symptoms among 104 patients followed for 27 months on average. Even in a later report on 116 patients with very severe AS (peak velocity ≥ 5.0 m/s), only 1 sudden death was observed during a median follow-up of 3.4 years. 145 Thus, sudden death may indeed occur even in the absence of preceding symptoms in patients with AS but appears to be a very uncommon event, with a rate of probably less than 1% per year during the asymptomatic phase of the disease. Finally, it has to be considered that sudden death has been reported even after successful valve replacement in patients with AS, at an incidence of about 0.3%, so the risk cannot be entirely eliminated by surgical treatment.146,147 Overall, a watchful waiting strategy, which consists of regularly following up patients as long as they are asymptomatic and referring them for surgery once they become symptomatic, results in a good survival that is not statistically different from that of an age- and gender-matched control population ( Figure 11-2). 144 FIGURE 11-2 Asymptomatic severe aortic stenosis. In initially asymptomatic patients, the rate of symptom onset ranges from less than 1% to 15% per year. Predictors of symptom onset include older age, male gender, AS severity, and functional status. One of the most important predictors of outcome in patients with AS is the degree of stenosis severity. The necessity for subsequent aortic valve surgery is directly related to peak aortic jet velocity over the whole spectrum of disease, with event rates being lowest in patients with mild stenosis, followed by those with moderate and then severe stenosis.47,144,148 The rate of symptom onset is about 8% per year in those with a jet velocity less than 3.0 m/s, 17% per year in those with a jet velocity of 3.0 to 4.0 m/s, and 40% per year in those with a jet velocity more than 4.0 m/s ( Figure 11-3). FIGURE 11-3 Impact of aortic jet velocity on outcome in asymptomatic aortic stenosis. Significant calcification, age, and the presence of CAD indicated higher event rates in patients with mild to moderate AS. 148 In addition, the rate of increase in aortic jet velocity over time is a strong predictor of clinical outcome.47,144,148–150 In severe asymptomatic AS, the rate of symptom onset is higher in patients older than 50 years and in those with significant valve calcification, suggesting that calcific disease progresses more rapidly than rheumatic aortic valve stenosis.144,151 Among 126 patients with asymptomatic severe AS, it was shown that the presence of a moderately to severely calcified aortic valve was associated with a significantly increased event rate, and 80% of these 126 patients experienced symptoms warranting aortic valve replacement or died within 4 years ( Figure 11-4). 144 The combination of a calcified aortic valve with a rapid hemodynamic progression, defined as an increase in peak aortic jet velocity of more than 0.3 m/s within 1 year, identified a patient group at particularly high risk, with an event rate of 79% within 2 years ( Figure 11-5). 144 The echocardiographic determination of aortic valve calcification has the advantage of being fast and easily obtainable at the moment of the echocardiographic exam. Although it is a semiquantitative method, the differentiation between no or mild and moderate to severe calcification can be easily made. The finding that aortic valve calcification is associated with a poor outcome was also confirmed by a study that assessed the degree of aortic valve calcification by electron-beam tomography. 152 Patients with very severe AS are also at an increased risk of experiencing a rapid symptom onset. Event-free survival rates at 3 years were found to 49%, 33%, and 11% for patients with peak aortic jet velocities between 4.0 and 5.0 m/s, between 5.0 and 5.5 m/s, and more than 5.5 m/s, respectively ( Figure 11-6). 145 Most patients with an aortic jet velocity higher than 5.0 m/s are already symptomatic at presentation, and those who are still asymptomatic have a high likelihood of a rapid symptom onset, in particular when their peak aortic jet velocity exceeds 5.5 m/s. These findings strongly support the need to define the entity of “very severe” AS. FIGURE 11-4 Effect of leaflet calcification on outcome in asymptomatic severe aortic stenosis.
Aortic Stenosis
Pathophysiology
Valvular Hemodynamics
Pressure Gradients
Valve Area
Other Indices of Stenosis Severity
Left Ventricular Pressure Overload
The Peripheral and Pulmonary Vasculature
Coronary Blood Flow
Exercise Physiology
Clinical Presentation
Clinical History
Physical Examination
Chest Radiography and Electrocardiography
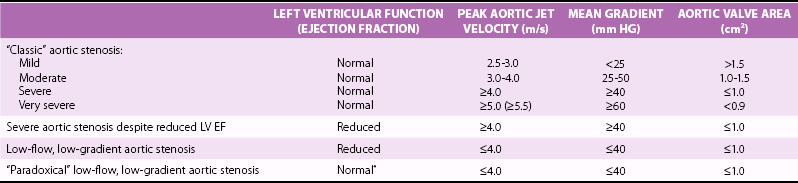
Echocardiography
Stress Testing
Cardiac Catheterization
Disease Course
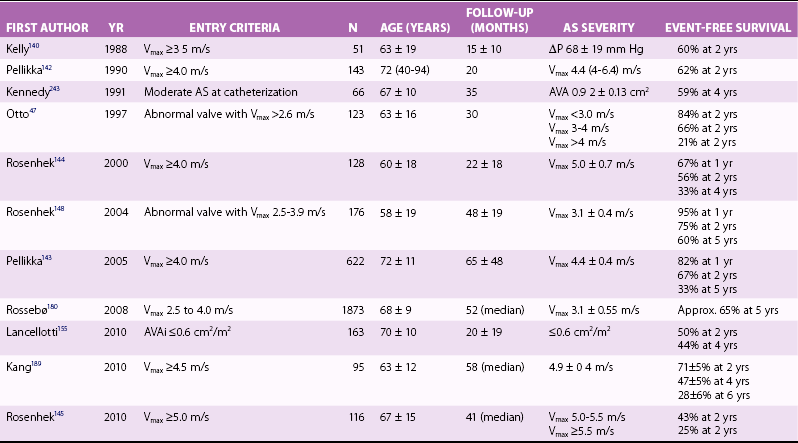
Clinical Outcome
Asymptomatic Patients
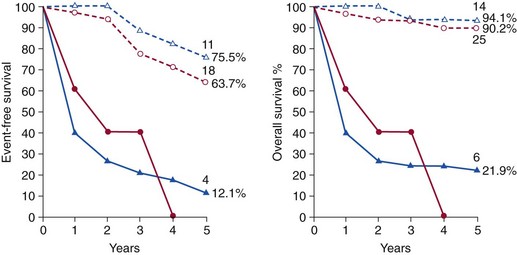
Survival curves are shown for hemodynamically severe aortic stenosis and combined lesions (triangles and blue lines) as well as aortic regurgitation (circles and red lines) in severely symptomatic (solid lines) and asymptomatic or mildly symptomatic (dashed lines) patients. Event free survival at 5 years, and the number of remaining subjects, are shown at the end of each line. (From Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987;8:471–83.)
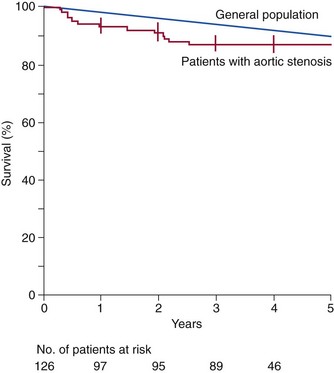
Kaplan-Meier estimates of overall survival among 126 patients with asymptomatic but severe aortic stenosis, as compared with age- and sex-matched persons in the general population. This analysis included perioperative and postoperative deaths among patients who required valve replacement during follow-up. The vertical bars indicate standard errors. (From Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611–7.)
Risk Stratification in Asymptomatic Patients
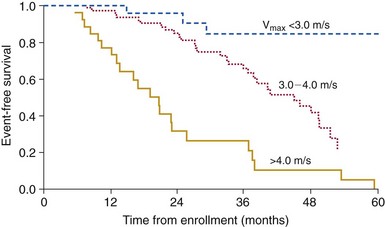
Cox regression analysis showing event-free survival in 123 initially asymptomatic adults with valvular aortic stenosis, defined by maximum aortic jet velocity (Vmax) at entry (P <0.001 by log-rank test). (From Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, et al. Prospective study of asymptomatic valvular aortic stenosis: clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95:2262–70.)
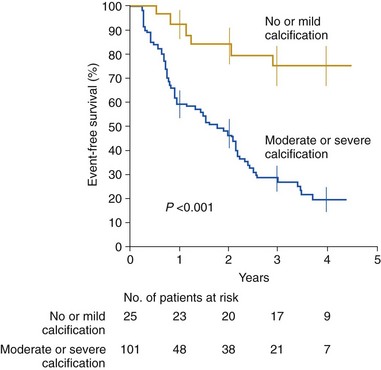
Kaplan-Meier estimates of event-free survival among 25 patients with no or mild aortic valve calcification compared with that among 101 patients with moderate or severe calcification. All patients had an aortic jet velocity of at least 4 m/s at study entry. The vertical bars indicate standard errors. (From Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611–7.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aortic Stenosis
Only gold members can continue reading. Log In or Register to continue









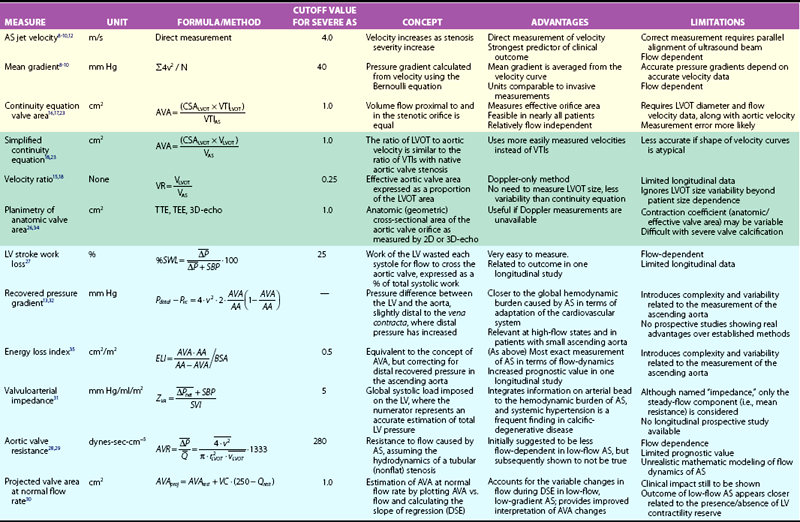
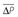 , mean transvalvular systolic pressure gradient; ELI, energy-loss coefficient; LVOT, left ventricular outflow tract; Pvc, pressure at the vena contracta; Q, mean systolic transvalvular flow-rate; Qrest, flow at rest; DSE, dobutamine stress echocardiography; r, radius; SBP, systolic blood pressure; Pdistal, pressure at the ascending aorta; SWL, stroke work loss; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; VTI, velocity-time integral; v, velocity; VC, valve compliance derived as the slope of regression line fitted to the AVA versus Q plot; VR, velocity ratio; ZVA, valvuloaortic impedance.
, mean transvalvular systolic pressure gradient; ELI, energy-loss coefficient; LVOT, left ventricular outflow tract; Pvc, pressure at the vena contracta; Q, mean systolic transvalvular flow-rate; Qrest, flow at rest; DSE, dobutamine stress echocardiography; r, radius; SBP, systolic blood pressure; Pdistal, pressure at the ascending aorta; SWL, stroke work loss; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; VTI, velocity-time integral; v, velocity; VC, valve compliance derived as the slope of regression line fitted to the AVA versus Q plot; VR, velocity ratio; ZVA, valvuloaortic impedance.