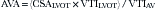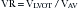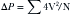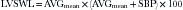2 Aortic Stenosis 
Goals of Echocardiography in Aortic Stenosis
 To determine that aortic stenosis is present
To determine that aortic stenosis is present
 To determine the level(s) of obstruction/stenosis (valvar versus other)
To determine the level(s) of obstruction/stenosis (valvar versus other)
 To determine the hemodynamic severity of the aortic stenosis
To determine the hemodynamic severity of the aortic stenosis
 To assess the regional and overall left ventricular (LV) function carefully
To assess the regional and overall left ventricular (LV) function carefully
 To determine the stroke volume
To determine the stroke volume
 To assess for bicuspid valve anomaly, its associations, and its complications
To assess for bicuspid valve anomaly, its associations, and its complications
Scanning Issues
Required Parameters to Obtain from Scanning
 Morphology (bicuspid?)—use a zoom view, focus in systole
Morphology (bicuspid?)—use a zoom view, focus in systole
 Severity of associated aortic insufficiency
Severity of associated aortic insufficiency
 Left ventricular outflow tract (LVOT) diameter
Left ventricular outflow tract (LVOT) diameter
 Ejection fraction (EF) or “grade” of dysfunction
Ejection fraction (EF) or “grade” of dysfunction
 Sinotubular junction (STJ) level (more pressure recovery when STJ is <30 mm)
Sinotubular junction (STJ) level (more pressure recovery when STJ is <30 mm)
 Ascending aorta level (associated dilation)
Ascending aorta level (associated dilation)
 Exclusion or identification of concurrent subvalvar obstruction
Exclusion or identification of concurrent subvalvar obstruction
 Noting height and weight for body surface area normalization
Noting height and weight for body surface area normalization
Scanning Notes
 To minimize error, a zoom view must be used.
To minimize error, a zoom view must be used.
 If the image quality confers ambiguity, repeat the measurement on several different zoom views.
If the image quality confers ambiguity, repeat the measurement on several different zoom views.
 LVOT is the single most important measurement: a 2-mm error confers a 20% aortic valve area (AVA) calculation error.
LVOT is the single most important measurement: a 2-mm error confers a 20% aortic valve area (AVA) calculation error.
 If the LVOT diameter is in doubt, consider:
If the LVOT diameter is in doubt, consider:
 Ensure that V1 Doppler sampling is correctly aligned—in some cases a more lateral apical sampling site is required to achieve acceptable alignment (within 20 degrees) if the LVOT long axis deviates markedly from that of the LV.
Ensure that V1 Doppler sampling is correctly aligned—in some cases a more lateral apical sampling site is required to achieve acceptable alignment (within 20 degrees) if the LVOT long axis deviates markedly from that of the LV.
 Do not record the V1 from within the LV cavity, or the recorded velocity and AVA tend to be too low.
Do not record the V1 from within the LV cavity, or the recorded velocity and AVA tend to be too low.
 Record the subvalvar V1 before the flow acceleration (easily depicted by the proximal isovelocity surface area [PISA]). Establishing the location of the PISA and sampling V1 to avoid the PISA is better technique than is arbitrarily placing the sample volume “1 cm beneath the aortic valve,” which may actually be within flow acceleration from the valvar stenosis or from concurrent subvalvar obstruction.
Record the subvalvar V1 before the flow acceleration (easily depicted by the proximal isovelocity surface area [PISA]). Establishing the location of the PISA and sampling V1 to avoid the PISA is better technique than is arbitrarily placing the sample volume “1 cm beneath the aortic valve,” which may actually be within flow acceleration from the valvar stenosis or from concurrent subvalvar obstruction.
 Although ideally the spectral envelope would be measured (planimetered) at the modal frequency (velocity), this is seldom discernable; therefore, planimetry is, for clinical purposes, performed on the outermost aspect of the spectral profile.
Although ideally the spectral envelope would be measured (planimetered) at the modal frequency (velocity), this is seldom discernable; therefore, planimetry is, for clinical purposes, performed on the outermost aspect of the spectral profile.
 AVA calculations traditionally are made from integrals; peak velocities are a surrogate.
AVA calculations traditionally are made from integrals; peak velocities are a surrogate.
 If sinus rhythm: measure three spectral profiles.
If sinus rhythm: measure three spectral profiles.
 If in atrial fibrillation: measure five spectral profiles.
If in atrial fibrillation: measure five spectral profiles.
 Ensure that the apical five-chamber and apical three-chamber V2 Doppler sampling views are correctly aligned (within 20 degrees). In some cases, a more lateral sampling site is needed.
Ensure that the apical five-chamber and apical three-chamber V2 Doppler sampling views are correctly aligned (within 20 degrees). In some cases, a more lateral sampling site is needed.
 If a concurrent subvalvar stenosis (LVOT velocity [V1] >1.5 m/sec) is likely, record the pre-subvalvar flow velocity, the pre-valvar velocity (V1), and also the V2. The modified Bernouilli equation should not be used if V1 is ≥1.5 m/sec.
If a concurrent subvalvar stenosis (LVOT velocity [V1] >1.5 m/sec) is likely, record the pre-subvalvar flow velocity, the pre-valvar velocity (V1), and also the V2. The modified Bernouilli equation should not be used if V1 is ≥1.5 m/sec.
 AVA calculations traditionally are made from integrals; peak velocities are a surrogate, although a reasonably accurate one.
AVA calculations traditionally are made from integrals; peak velocities are a surrogate, although a reasonably accurate one.
 If sinus rhythm: measure three spectral profiles.
If sinus rhythm: measure three spectral profiles.
 If in atrial fibrillation: measure five spectral profiles.
If in atrial fibrillation: measure five spectral profiles.
 If in the idea world: measure ten spectral profiles.
If in the idea world: measure ten spectral profiles.
 Annotate the site of sampling for future reference comparison.
Annotate the site of sampling for future reference comparison.
 Verify whether there is/is not concurrent intracavitary flow acceleration (subaortic valve velocity ≥ 1.5 m/sec), as discussed in the following section.
Verify whether there is/is not concurrent intracavitary flow acceleration (subaortic valve velocity ≥ 1.5 m/sec), as discussed in the following section.
 A narrow aortic root (STJ ≤ 30 mm): a narrow aorta facilitates the “pressure recovery” phenomenon that detects a higher gradient than the recovered gradient measured by catheterization.
A narrow aortic root (STJ ≤ 30 mm): a narrow aorta facilitates the “pressure recovery” phenomenon that detects a higher gradient than the recovered gradient measured by catheterization.
Equations
where AVG = average gradient, CSA = cross-sectional area, SBP = systolic blood pressure, and VTI = velocity time integral.
Pathophysiology and Findings of Aortic Stenosis
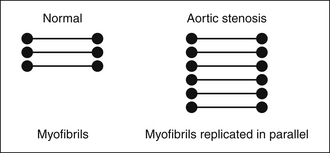
In severe AS, where the systolic pressure within the LV doubles, LV mass essentially doubles (increases to 178 g/m2 vs. normal of 86 g/m2),1 due to near doubling of the wall thickness to normalize wall stress that otherwise would be nearly doubled (Fig. 2-1).
Severe AS may remain seemingly static and compensated for years, or may progress. The rate of progression depends on many factors, few of which are well understood. Progression rates between –0.01 cm2/year and –0.1 cm2/year have been described. Calcification of the aortic valve is somewhat predictive of a faster rate of progression, but it is not known how to quantify calcification toward this purpose. Electron beam CT studies have not shown good correlation of calcification quantification by Agatston score with aortic valve area, especially for moderate and severe AS.2
In summary, the pathophysiology of severe AS generates expected findings: increased wall thickness, no increase in left ventricular cavitary size, and left atrial dilation. An increase in cavitary size requires additional explanation, such as concurrent volume overload from aortic insufficiency or mitral regurgitation, impaired systolic function from CAD or cardiomyopathy, or terminal decompensated AS.
Role of Transesophageal Echocardiography in Aortic Stenosis
There is no routine role for TEE in the assessment of AS.
TEE probably is the best test available to image subvalvar lesion morphology in detail.
TEE is a standard test prior to percutaneous aortic valve replacement (AVR) (see Chapter 26) and optimally is used to determine the size of the prosthesis and the morphology of the annuli, valve, and root.
Reporting Issues
Gradient Issues
 Bradycardia in the context of normal LV function or contractile recruitment
Bradycardia in the context of normal LV function or contractile recruitment
 Causes of larger stroke volume ± lower peripheral resistance
Causes of larger stroke volume ± lower peripheral resistance
 Undersampling of the V2 spectral profile is a common clinical problem, as the spectral profile boundary may be vague.
Undersampling of the V2 spectral profile is a common clinical problem, as the spectral profile boundary may be vague.
 Fewer than 5% of cases of AS will have inadequate spectral profiles; the percentage is very much determined by the time and effort made to acquire the spectral recording.
Fewer than 5% of cases of AS will have inadequate spectral profiles; the percentage is very much determined by the time and effort made to acquire the spectral recording.
 Adequate spectral profiles are complete and plausible parabolic profiles.
Adequate spectral profiles are complete and plausible parabolic profiles.
 If the peak velocity is to be marked with cross-hairs, the cross-hairs should not be placed on the peak of a spectral profile, because that may falsely confer an impression of the true peak to the reviewer. The cross-hairs should be placed off to the side of the presumed peak to allow the reviewing or reporting physician to establish independently that the peak was true, as a useful internal check.
If the peak velocity is to be marked with cross-hairs, the cross-hairs should not be placed on the peak of a spectral profile, because that may falsely confer an impression of the true peak to the reviewer. The cross-hairs should be placed off to the side of the presumed peak to allow the reviewing or reporting physician to establish independently that the peak was true, as a useful internal check.
 If the profile initially does not appear complete, persistence in attempting better ones is required, as there is a (albeit diminished) return. Techniques that should be used include the following:
If the profile initially does not appear complete, persistence in attempting better ones is required, as there is a (albeit diminished) return. Techniques that should be used include the following:
 The published correlation of Doppler versus catheterization mean gradients averages 0.90,3,4 but importantly, 1 standard error of estimate (SEE) of Doppler gradient versus catheterization gradient is actually 10 mm Hg [SEE range, 6–12 mm Hg].5
The published correlation of Doppler versus catheterization mean gradients averages 0.90,3,4 but importantly, 1 standard error of estimate (SEE) of Doppler gradient versus catheterization gradient is actually 10 mm Hg [SEE range, 6–12 mm Hg].5
 Rahimtoola5 emphasized consideration of the SEE by Doppler when describing AS severity (by gradient):
Rahimtoola5 emphasized consideration of the SEE by Doppler when describing AS severity (by gradient):
 Recall that the pressure recovery phenomenon at aortic root level is seen with small aortic roots (dimensions <30 mm) and restrictive planar divergent orifices. Pressure recovery may add 15% to 30% to the gradient.
Recall that the pressure recovery phenomenon at aortic root level is seen with small aortic roots (dimensions <30 mm) and restrictive planar divergent orifices. Pressure recovery may add 15% to 30% to the gradient.
 LVSWL, originally a catheterization-based parameter, can be approximated by echocardiography, and has been validated as a way to discriminate clinical end-points: an LVSWL ≤25 is the best predictor.6 The concept expresses kinetic energy loss across the aortic valve in reference to the aortic pressure.
LVSWL, originally a catheterization-based parameter, can be approximated by echocardiography, and has been validated as a way to discriminate clinical end-points: an LVSWL ≤25 is the best predictor.6 The concept expresses kinetic energy loss across the aortic valve in reference to the aortic pressure.
Continuity Equation–Derived Aortic Valve Area Issues
 Place less emphasis on the valve area alone; instead, emphasize the combination of area, gradient, and stroke volume. Unless these afford a congruent assessment of aortic stenosis severity, further consideration is needed.
Place less emphasis on the valve area alone; instead, emphasize the combination of area, gradient, and stroke volume. Unless these afford a congruent assessment of aortic stenosis severity, further consideration is needed.
 Continuity-derived AVA is not flow independent.7
Continuity-derived AVA is not flow independent.7
 The multiple variables used in the calculation of AVA may allow for introduction of considerable composite error.
The multiple variables used in the calculation of AVA may allow for introduction of considerable composite error.
 The average published correlation of Doppler-derived AVA versus catheterization-derived AVA is r = 0.90. However, recall that the SEE of Doppler AVA versus catheterization gradients is, on average, 0.3 mm Hg [0.1–0.4 cm2].5
The average published correlation of Doppler-derived AVA versus catheterization-derived AVA is r = 0.90. However, recall that the SEE of Doppler AVA versus catheterization gradients is, on average, 0.3 mm Hg [0.1–0.4 cm2].5
 Rahimtoola5 suggests consideration of patient body size indexing of the AVA.
Rahimtoola5 suggests consideration of patient body size indexing of the AVA.
 Report AVA only to a single decimal place. A calculation accurate at best to ± 0.3 cm2 cannot be realistically described to a second decimal place.
Report AVA only to a single decimal place. A calculation accurate at best to ± 0.3 cm2 cannot be realistically described to a second decimal place.
Other Issues
Describe the dimensions and appearance of the root and ascending aorta. These are potentially important surgical details that may modify the approach to surgery. Furthermore, if a case is obviously severe, and the aortic annulus is small, the size of the annulus should be mentioned. An unusually small root will allow only a small prosthesis, which engenders a gradient little better than the AS it was supposed to relieve—an outcome known as “patient–prosthesis mis-match.” Although such a mismatch probably is not as significant a clinical problem as has been purported, it still has validity in some cases.
Concurrent Subvalvar Stenosis
 Subvalvar AS is revealed by the PISA (flow convergence) at a level well beneath the aortic valve (>1 cm beneath, but influenced by the Nyquist limit). Formerly, emphasis of detection was on pulsed wave Doppler sampling; however, as color flow mapping is essentially a flow map of pulsed-wave Doppler, clear color Doppler depiction of PISA well away from the valve is equivalent.
Subvalvar AS is revealed by the PISA (flow convergence) at a level well beneath the aortic valve (>1 cm beneath, but influenced by the Nyquist limit). Formerly, emphasis of detection was on pulsed wave Doppler sampling; however, as color flow mapping is essentially a flow map of pulsed-wave Doppler, clear color Doppler depiction of PISA well away from the valve is equivalent.
 A two-fold focal step-up in velocity and a “dagger” or tooth shape of the profile are consistent with a intracavitary stenosis.
A two-fold focal step-up in velocity and a “dagger” or tooth shape of the profile are consistent with a intracavitary stenosis.
 Provocative maneuvers (e.g., posture change, Valsalva maneuver, leg raise) may reveal the subvalvar gradient to be dynamic.
Provocative maneuvers (e.g., posture change, Valsalva maneuver, leg raise) may reveal the subvalvar gradient to be dynamic.
 Zoom views are helpful to localize the step-up, and begin to resolve its nature, e.g., muscular, membrane, or tunnel, or from multiple causes.
Zoom views are helpful to localize the step-up, and begin to resolve its nature, e.g., muscular, membrane, or tunnel, or from multiple causes.
 TEE is superbly able to define the anatomy responsible for subvalvar obstruction; cardiac MRI is very good, and cardiac CT likely is as well.
TEE is superbly able to define the anatomy responsible for subvalvar obstruction; cardiac MRI is very good, and cardiac CT likely is as well.
 Although one catheter study has suggested a 30% incidence of subvalvar gradients,8 the incidence does not appear to be as high as that.
Although one catheter study has suggested a 30% incidence of subvalvar gradients,8 the incidence does not appear to be as high as that.
 In the presence of subvalvar AS, the routine sampling of V1 will fall into the flow convergence/flow acceleration or vena contracta of subvalvar stenosis. If the subvalvar velocity is >1.5 m/sec, the calculations of aortic valve area are skewed.
In the presence of subvalvar AS, the routine sampling of V1 will fall into the flow convergence/flow acceleration or vena contracta of subvalvar stenosis. If the subvalvar velocity is >1.5 m/sec, the calculations of aortic valve area are skewed.
Low-Gradient Severe Aortic Stenosis
 The definition of “low-gradient severe AS” is unresolved and variable, but may be approached as follows:
The definition of “low-gradient severe AS” is unresolved and variable, but may be approached as follows:
 The challenge is to distinguish patients with end-stage AS with failing LV systolic function (responsible for the low gradient) from patients with moderate AS with poor LV systolic function, in whom a factor other than AS is responsible for the low gradient.
The challenge is to distinguish patients with end-stage AS with failing LV systolic function (responsible for the low gradient) from patients with moderate AS with poor LV systolic function, in whom a factor other than AS is responsible for the low gradient.
 Low-gradient severe AS can be discounted by a convincingly normal stroke volume: if the gradient is low and the stroke volume is normal, then low-gradient severe AS is unlikely to be present.
Low-gradient severe AS can be discounted by a convincingly normal stroke volume: if the gradient is low and the stroke volume is normal, then low-gradient severe AS is unlikely to be present.
 Citing low EF% or “grade” to describe low-output AS is not adequate. The stroke volume should be shown to be low to establish that the per-beat output is low, and potentially responsible for the low gradient. There may be normal stroke volume with a low EF% LV (if dilated) and low stroke volume with an LV with normal EF% (if it is under-loaded or if there is severe MR). Therefore, LV grade is not synonymous with output.
Citing low EF% or “grade” to describe low-output AS is not adequate. The stroke volume should be shown to be low to establish that the per-beat output is low, and potentially responsible for the low gradient. There may be normal stroke volume with a low EF% LV (if dilated) and low stroke volume with an LV with normal EF% (if it is under-loaded or if there is severe MR). Therefore, LV grade is not synonymous with output.
 EF% generally increases after AVR10,11 unless a perioperative infarction occurs, or the LV is intractably stiff. It appears that selected cases of low-gradient severe AS still benefit from AVR,12 although the data in this field are preliminary.13
EF% generally increases after AVR10,11 unless a perioperative infarction occurs, or the LV is intractably stiff. It appears that selected cases of low-gradient severe AS still benefit from AVR,12 although the data in this field are preliminary.13
 In the presence of low output, both the Gorlin catheterization formula11,14 and continuity methods are less accurate in the estimation of AVA.
In the presence of low output, both the Gorlin catheterization formula11,14 and continuity methods are less accurate in the estimation of AVA.
 When catheterizing suspected low-gradient AS, use of either two catheters or double-lumen catheters should be considered to eliminate phase shift artifacts, which can introduce a difference of >10 mm Hg in the gradient recording from a femoral side-arm.
When catheterizing suspected low-gradient AS, use of either two catheters or double-lumen catheters should be considered to eliminate phase shift artifacts, which can introduce a difference of >10 mm Hg in the gradient recording from a femoral side-arm.
 Low-gradient AS should be reported as a possibility or probability, depending on how strongly it is believed to be present.
Low-gradient AS should be reported as a possibility or probability, depending on how strongly it is believed to be present.
 Valve resistance has been proposed as a means of establishing the severity of AS (>300 dynes/sec/cm−5 = severe disease) that is independent of flow output, but it has not been proved to be better than valve area calculation,9 nor has it been popular. It appears less useful than LVSWL.6
Valve resistance has been proposed as a means of establishing the severity of AS (>300 dynes/sec/cm−5 = severe disease) that is independent of flow output, but it has not been proved to be better than valve area calculation,9 nor has it been popular. It appears less useful than LVSWL.6
 Determination of contractile reserve in low-gradient severe AS cases
Determination of contractile reserve in low-gradient severe AS cases
 In cases of suspected low-gradient severe AS, particular scrutiny should be afforded to the size of the aortic annulus/root. If the root is unusually small (e.g., 19–20 mm), then the size of prosthesis that would be implanted would confer a moderate gradient, which might be little different from the preoperative gradient.
In cases of suspected low-gradient severe AS, particular scrutiny should be afforded to the size of the aortic annulus/root. If the root is unusually small (e.g., 19–20 mm), then the size of prosthesis that would be implanted would confer a moderate gradient, which might be little different from the preoperative gradient.
Discordance of Catheterization and Echocardiographic Determination of the Severity of Aortic Stenosis
Possible Reasons for Discordance of Catheterization and Echocardiographic Determination of the Severity of Aortic Stenosis
 Recall that nonsimultaneous (echocardiographic and catheterization) estimates of gradient and area are obviously likely to differ more as stroke volume (cardiac output) and peripheral load is variable over time.
Recall that nonsimultaneous (echocardiographic and catheterization) estimates of gradient and area are obviously likely to differ more as stroke volume (cardiac output) and peripheral load is variable over time.
 Recall that the published SEE of Doppler gradients versus catheterization gradients averages 10 mm Hg.
Recall that the published SEE of Doppler gradients versus catheterization gradients averages 10 mm Hg.
 Recall that the published SEE of Doppler AVA versus catheterization gradients is about 0.3 cm2.
Recall that the published SEE of Doppler AVA versus catheterization gradients is about 0.3 cm2.
 Recall that the following invariably reduce the accuracy of echocardiographic estimates of AVG and AVA:
Recall that the following invariably reduce the accuracy of echocardiographic estimates of AVG and AVA:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree