Essentials of Diagnosis
General Considerations & Etiology
Over the past century, there has been a linear climb in record life expectancy and an overall aging of the human race. Therefore, we are faced with an epidemic of aging and age-associated disease, not the least of which is valvular heart disease. Aortic stenosis, the narrowing of the aortic valve orifice caused by failure of the leaflets to open normally, is now the most common indication for valve replacement in North America and Europe.
The pathogenesis of aortic stenosis is most commonly progressive calcification and degeneration of a trileaflet or congenitally bicuspid valve. Although once thought to be a degenerative process, it is now recognized that calcific aortic stenosis is in fact an active disease process that shares similarities to atherosclerosis where there is inflammation, lipid accumulation, and calcification of the leaflets. The mechanisms by which some valves degenerate and become stenotic while others remain relatively normal are unknown but are probably related to genetic polymorphisms. Those with end-stage renal disease, Paget disease, or severe familial hypercholesterolemia may present with calcific aortic stenosis at a younger age and are susceptible to more rapid progression of stenosis severity.
Rheumatic valve disease is a rare cause of aortic stenosis in industrialized nations. However, for indigenous populations within these countries as well as in developing countries, there remains a significant prevalence of rheumatic valve disease. In contrast to calcific aortic valve stenosis, the rheumatic valve shows adhesion, leaflet retraction, and commissural fusion. Along or just a few millimeters away from the free margins of the valve leaflets, small sessile nodules develop that also contribute to leaflet malcoaptation. Therefore, the rheumatic aortic valve invariably will leak. Rheumatic aortic valve disease is almost never present in isolation, and there is invariably concomitant mitral valve disease. A patient with aortic stenosis and a perfectly normal mitral valve should be considered as having another cause for their disease. Other rare causes of aortic stenosis include those associated with connective tissues diseases such as systemic lupus erythematosus and ochronosis.
Clinical Findings
Those with acquired aortic stenosis generally have a long latent period before the onset of the salient clinical manifestations of the disease: effort-related dyspnea (heart failure), angina, and syncope. The most common initial clinical manifestations are a gradual decline in functional capacity and effort-related dyspnea. Regardless of the initial presenting symptom(s), it is imperative to ensure that the pathophysiologic mechanism is attributed to valve disease and not another mechanism such as concomitant coronary artery or lung disease because the onset of even mild symptoms attributed to aortic stenosis heralds a dramatic increase in the mortality rate for these patients if the valve is not replaced. Symptoms are therefore the guidepost for intervention, and understanding them is the key to understanding and managing the disease.
The primary determinants of left ventricular systolic function are contractility and afterload. The load on individual myocardial fibers can best be described as left ventricular wall stress and defined by the Laplace equation:
With acquired aortic stenosis, the obstruction will commonly progress gradually over time. Left ventricular adaptation to increases in systolic pressure is the parallel replication of sarcomeres to increase its thickness (concentric hypertrophy) in an effort to normalize wall stress and maintain systolic performance. As the severity of stenosis progresses, the increase in wall thickness may become insufficient to offset the rise in pressure (“afterload mismatch”), resulting in a rise in wall stress and a decline in ventricular function. The presence of aortic stenosis may also result in true depression of myocardial contractility, the exact mechanism of which is unclear but likely related to a loss of contractile elements secondary to reduced coronary blood flow. Thus a decline in ejection fraction results from the interplay of varying degrees between excessive afterload and true myocardial depression. Those in whom the decline in function is largely attributed to afterload mismatch are more likely to restore ventricular function following aortic valve replacement. The evaluation of left ventricular ejection phase indices should be related to the global wall tension; however, clinically it is very difficult to tease out the extent by which wall stress (afterload) and contractility are contributing to a noted decline in ejection fraction.
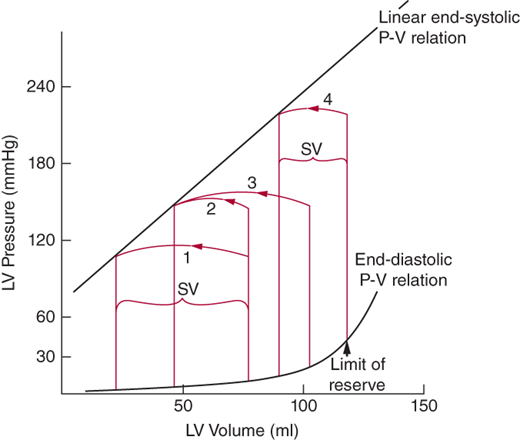
The increase in left ventricular wall thickness, although imposed in an effort to maintain wall stress and systolic performance, does have maladaptive physiologic consequences. The increase in wall thickness makes it harder to fill the ventricle, and therefore, higher filling pressures are required to achieve any given volume, and end-diastolic volume will be required to increase to allow the maintenance of a normal ejection fraction (a rightward shift in the ventricular diastolic pressure volume relationship) (Figure 17–1). In alignment with the concepts of continuity disease, when the mitral valve opens, the left atrium is now exposed to the hemodynamic milieu of the left ventricle. The increased ventricular diastolic pressure is transmitted to atrium and further back into the pulmonary veins and lungs, resulting in pulmonary congestion and an increased work of breathing. Additionally, with outflow obstruction, there is a prolongation of the ejection phase; this in conjunction with an exercise-induced increase in heart rate will result in a reduction of the diastolic filling time, and the ventricle will reach its limit of “preload reserve.” Once limits of preload reserve are met, stroke volume now becomes directly related to ventricular pressure (see Figure 17–1), resulting in an inability to increase cardiac output and further contributing to the presence of dyspnea and exercise intolerance.
Figure 17-1
Left ventricular (LV) pressure-volume (P-V) loops. Loop 1 is normal. As LV pressure increases, stroke volume (SV) would decrease if end-diastolic volume were fixed (loop 2). The ventricle, however, adapts by increasing end-diastolic volume to maintain a normal SV (loop 3). Eventually, the limits of preload reserve are met, whereby increases in LV pressure result in a decline in SV (SV is now directly related to LV pressure; loop 4). (Adapted with permission from Ross J. J Am Coll Cardiol. 1985;5[4]:811–26.)
Angina results from myocardial ischemia, which occurs when there is an imbalance between myocardial oxygen requirements/demand and myocardial oxygen supply. Although epicardial coronary artery disease often coexists with aortic stenosis, symptoms of angina frequently occur in those without epicardial coronary artery disease. Oxygen demand is best estimated clinically by the product of heart rate and wall stress, and as noted earlier, eventually the extent of hypertrophy cannot keep up with pressure demands of the ventricle and wall stress increases. In the absence of epicardial coronary artery disease, myocardial oxygen supply may also be decreased secondary to the rise in left ventricular end-diastolic pressure and a delayed rate of ventricular relaxation, which impairs coronary “diastolic suction,” both contributing to reduced coronary perfusion and a decline in coronary flow reserve needed to offset increased oxygen demands during stress or exercise. In addition, with exercise and increased heart rates, there is reduced diastolic coronary perfusion time.
Syncope is a transient loss of consciousness due to cerebral hypoperfusion. Syncope in those with aortic stenosis usually occurs during exercise. There are two primary mechanisms, not mutually exclusive, which have been theorized to cause syncope. First, the narrowed aortic valve does not permit the appropriate increase in cardiac output necessary to offset the associated reduction in total peripheral resistance associated with exercise, resulting in a drop in blood pressure. Second, the very high ventricular pressure that develops with exercise when sensed by ventricular mechanoreceptors triggers a reflexive vasodepressor response, also leading to a decline in blood pressure. Less commonly, exercise can result in either ventricular or supraventricular arrhythmias, which can lead to a reduction in cardiac output and consequently a drop in blood pressure.
The character of a murmur can be described by its timing and shape, intensity and pitch, and location and radiation, along with changes in these characteristics imposed by transient changes in intracardiac hemodynamics.
The murmur of valvular aortic stenosis is a midsystolic murmur, which begins after the first heart sound and ends before the aortic (A2) component of the second heart sound. During systole, as blood flow velocity accelerates across the valve, the intensity of the murmur increases, and as blood flow velocity decelerates, the intensity diminishes. Therefore there is a classic “crescendo-decrescendo” configuration or shape to the murmur of valvular aortic stenosis. Further insights into the configuration of the murmur may help to discern the severity of disease, where the later in systole the peak intensity occurs, generally the stenosis severity is more severe.
The intensity of the aortic stenosis murmur relates to the quantity and velocity of transaortic blood flow along with the ability to transmit sound through the chest. The pitch of the murmur relates to the pressure gradient and size of the “aperture” with which the blood flows through. In general, as the stenosis severity increases, the intensity and pitch of the murmur will increase. However, in those with a thick ventricle and low end-diastolic volume or in the presence of a decline in cardiac function, both of which may result in a low stroke volume, the intensity and pitch of the murmur may be lower than expected for the given severity of stenosis. Additionally, the ability to transmit the sound through the chest is impaired in those with a pericardial effusion, emphysema, or obesity. Therefore, one should be cautious in excluding significant aortic stenosis solely based on murmur intensity or pitch.
The murmur of aortic stenosis typically is heard loudest in the “aortic area,” the right second interspace at the sternal border, and will often radiate into the carotids and along a line from the aortic area toward the left ventricular apex. At the apex, the intensity and pitch of the murmur may change to more resemble those of the murmur of mitral regurgitation; however, the shape and configuration of the murmur do not change. This is known as the Gallavardin phenomenon.
A transient alteration in intracardiac hemodynamics may influence the characteristics of a murmur, helping to further specify its etiology.
- Isometric handgrip: Increases systemic vascular resistance and blood pressure, decreasing the intensity of the murmur of aortic stenosis.
- Standing/Valsalva maneuver (phase II): Decreases ventricular filling, reducing the intensity of the murmur of aortic stenosis and most all other valve murmurs except for that of hypertrophic cardiomyopathy and the duration of the murmur of mitral valve prolapse.
- Squatting: Increases ventricular filling but also increases systemic vascular resistance. Its effect on the murmur of aortic stenosis is therefore generally neutral; however, it will reduce the intensity of the murmur of hypertrophic cardiomyopathy.
- Cycle length: The intensity of the murmur of aortic stenosis may vary from beat to beat in those with atrial fibrillation or premature contractions, increasing in intensity in the beats following a longer R-R interval where diastolic filling time is increased.
The carotid artery pulse wave contour in those with aortic stenosis is characterized as small and slow rising (parvus et tardus). However, in older individuals with reduced arterial compliance, this finding is abrogated by increased reflected waves in the aorta. One may also appreciate a carotid shudder.
The presence of significant aortic stenosis may result in paradoxical splitting of the second heart sound where the A2 component of S2 is delayed, resulting in S2 being more split during expiration than inspiration. Reduced movement of the aortic valve may render A2 inaudible where only a single second heart sound can be appreciated.
As noted earlier, left ventricular hypertrophy imposed by the increase in wall stress reduces ventricular wall compliance. The fourth heart sound is a low-pitch sound heard coincident with late diastolic filling, due to atrial contraction, in a ventricle with reduced wall compliance and increased end-diastolic pressure.
The apical impulse, which in aortic stenosis is generally also the point of maximal impulse, remains in its normal position but is sustained due to prolongation of the ejection time. The atrial component of ventricular filling may also be palpable. With simultaneous palpation of the apical and carotid impulses, normally one will appreciate little delay in their peaks; however, they become separated in time proportional to the severity of stenosis.
The clinical severity of aortic stenosis is largely an operational classification based on the presence or absence of symptoms, as discussed earlier. However, the indications for consideration of either a surgical or percutaneous aortic valve replacement rely on an estimate of stenosis severity.
Left ventricular hypertrophy is the primary finding noted in those with aortic stenosis. Other common findings include left atrial abnormality and ST- and T-wave abnormalities. There are, however, no electrocardiogram findings that are either sensitive or specific for aortic stenosis.
With isolated aortic stenosis, the cardiac silhouette is generally normal in size with possible rounding of the left heart border consistent with concentric left ventricular hypertrophy. There may be signs of left atrial enlargement and pulmonary venous hypertension. The aortic shadow may become enlarged, and valve calcification may be appreciated.
Echocardiography is the principal clinical tool used for the evaluation of aortic stenosis. Appropriate use criteria deem echocardiography appropriate when clinical evaluation provides “reasonable suspicion of valvular or structural heart disease or re-evaluation of known valvular heart disease with a change in clinical status or cardiac exam or to guide therapy.” Routine surveillance in the absence of a change in clinical status or cardiac examination is deemed appropriate every 3 years in those with mild and every 1 year in those with moderate to severe valvular stenosis. The echocardiogram should include not only anatomic and hemodynamic measures of stenosis severity but also an assessment of the left ventricular response to the pressure load, insights into whether there is dilation of the ascending aorta, and assessment for the presence of coexisting valve regurgitation and other cardiac abnormalities.
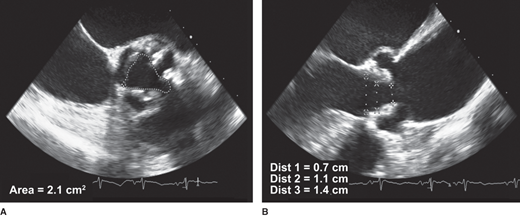
Although most outcome data in those with aortic stenosis are based on measures of the hemodynamic severity and physiologic orifice area, the anatomic evaluation of the valve is gaining increased importance in the evolving era of percutaneous valve replacement in guiding patient selection and procedural planning. From primarily the transthoracic parasternal views, or transesophageal imaging if the transthoracic images are suboptimal, the number of leaflets, extent of calcification, leaflet thickening, and mobility should be evaluated. An anatomic measure of the geometric valve area can be obtained by planimetry. The fundamental limitations to accurate and reproducible measurements of the geometric orifice area are image attenuation secondary to leaflet calcification and the spatial integration of images required to ensure planimetry of the minimal opening area at the leaflet tips (Figure 17–2). It is for these reasons that the measure of geometric orifice area is reserved clinically for those circumstances where Doppler measurements are unreliable.
Figure 17-2
Planimetry of the aortic valve. A: Short axis image of the aortic valve and a calculated anatomic valve area by planimetry. Leaflet calcification and image attenuation limit accurate delineation of the leaflet borders. B: Long axis image of the aortic valve noting that slight alteration in the tomographic slice obtained through the aortic valve will result in marked variation in the valve area calculated by planimetry.
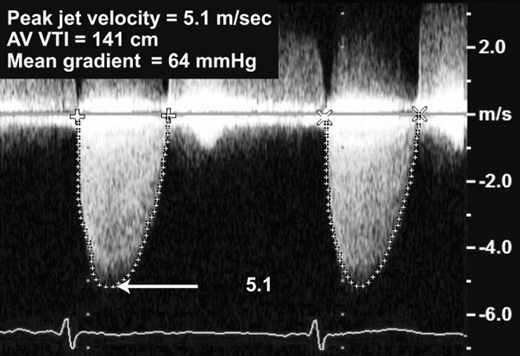
The principal measures of the hemodynamic severity include peak transaortic jet velocity, peak instantaneous and mean pressure gradients, and valve area (effective orifice area by the continuity equation).
The principle of conservation of energy states that the total amount of energy in a closed system remains constant. Energy can change its location and form but can be neither created nor destroyed. With respect to flow, as the flow stream approaches a narrowed orifice, its kinetic energy increases and potential energy decreases. Distal to the narrowed orifice, pressure is lost in part due to the dissipation of kinetic energy as heat. This creates a pressure gradient across the valve orifice. Continuous wave Doppler is used to determine the maximum jet velocity through the stenotic valve. Meticulous imaging from multiple acoustic windows is required to ensure that flow velocities are acquired with a parallel intercept angle to the direction of flow limiting the error of underestimation of the peak velocity. The Bernoulli equation is then applied to the highest jet velocity obtained to calculate the peak instantaneous gradient (Figure 17–3).